You are here: Home: LCU 4 | 2005 : Mark A Socinski, MD
Mark A Socinski, MD |
EDITED COMMENTS |
 ECOG-E4599: Phase III randomized trial of carboplatin/paclitaxel with or without bevacizumab ECOG-E4599: Phase III randomized trial of carboplatin/paclitaxel with or without bevacizumab
One of the biggest stories presented at ASCO 2005 was that of bevacizumab in the ECOGE4599 trial (Sandler 2005). The trial design was simple — paclitaxel plus carboplatin with or without bevacizumab. E4599 was based on impressive, favorable findings from a Phase II experience, which were published last year in the JCO (Johnson 2004).
A review of the eligibility criteria for the Phase III trial is important because the population studied had nonsquamous cell tumors, no brain metastases, a PS from 0-1, no anticoagulation therapy and no recent history of hemoptysis. Therefore, this was a clinically selected population of patients, first and foremost for safety issues.
Response rates and survival benefits
In the control arm, paclitaxel plus carboplatin alone was associated with a median survival of just over 10 months, which is more favorable than the median survival of approximately eight months for paclitaxel plus carboplatin in E1594 (Schiller 2002). In the investigational arm, the addition of bevacizumab to paclitaxel plus carboplatin resulted in significant improvements in both median and progression-free one-year survival of approximately two months and an approximate eight percent increase in absolute one-year survival.
One-year survival in the bevacizumab arm was just over 50 percent, which is a major milestone in terms of one-year survival in NSCLC. The response rate in the control arm of ECOG-E4599 was approximately 10 percent and approximately 27 percent in the bevacizumab arm.
Toxicity
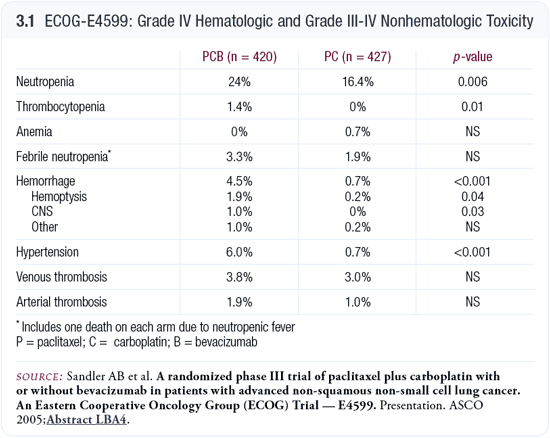
In the bevacizumab arm, slightly more myelosuppression occurred. An approximate 4.5 percent incidence of hemorrhagic complications also occurred — mostly hemoptysis — compared to an incidence of 0.8 percent in the control arm, which translates to roughly a five-fold increase in risk (Sandler 2005; [3.1]). We have to learn how to identify patients at high risk. A review of which patients suffered the fatal hemorrhagic complications could provide insight into how these patients were different from others.
We must be careful extrapolating these data to the general population, because the results suggest that hemorrhagic complications may occur in one of every 20 or 25 patients who are treated. As we take the positive findings from E4599 and integrate them into everyday practice, we must be as selective in treating patients as the eligibility criteria were in the trial for determining to whom we offer this regimen as a standard of care.
Potential risk factors for bevacizumab-associated bleeding
Patients with large central lesions who possibly undergo early cavitation may have a heightened risk for bleeding complications. The CT scans of such patients provide a sense that the tumor might be making up part of the pulmonary arterial wall or that it might be adjacent to another vital vascular structure. It’s not known whether bevacizumab should be discontinued in these patients. We must use our best clinical judgment, as no clear guidelines exist for these circumstances.
Bleeding complications might be related to the manifestation of an anti-VEGF effect or a profound tumor response. The other issue that has been frequently discussed involves determining the unique predisposing aspects of the squamous cell tumor population. We know that the squamous cell population tends to have more centrally located tumors and perhaps more bulky central tumors that are associated with increased local invasion.
A patient with a central, bulky adenocarcinoma that appears similar radiographically to a squamous cell tumor may have the same risk of bleeding as those with squamous cell tumors. I’m not convinced that increased bleeding complications relate to histology; rather, the anatomical location of tumors may play an important role.
Combining bevacizumab with erlotinib
The concept of using two targeted agents, such as anti-EGFR and anti-VEGF agents, is intriguing. Over the past year, the BR21 data have validated the EGFR pathway as significant and therapeutic (Shepherd 2004). The angiogenesis pathway has also emerged as an important pathway in lung cancer. Therefore, targeting both pathways makes sense.
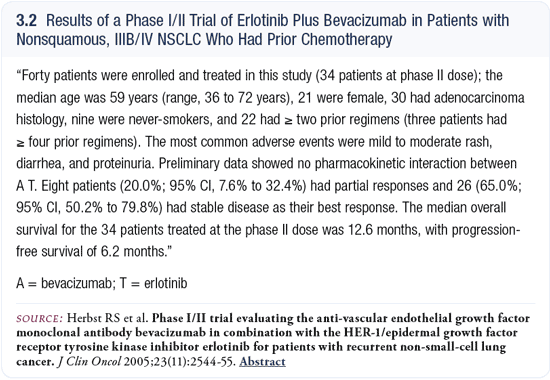
Drs Sandler and Herbst reported interesting Phase I/II data that described a 20 percent response rate and favorable survival outcomes among patients who received erlotinib and bevacizumab (Herbst 2005; [3.2]). Once again, the caveat was that the study group was comprised of a select group of patients with nonsquamous cell tumors and no brain metastases. Both bevacizumab and erlotinib may be administered at full doses. Grade III or IV toxicities were not observed using erlotinib plus bevacizumab.
If bevacizumab is used following chemotherapy in the first-line setting, one might argue that it would be appropriate to add erlotinib, which is FDA approved in the second-line setting. By initiating this regimen soon after chemotherapy, you may be instituting your second-line therapy earlier. This should be studied to compare the value of earlier therapy versus delayed treatment in the second-line setting. Another question is: In a patient receiving maintenance bevacizumab, should bevacizumab be discontinued, or should erlotinib be added to the bevacizumab upon disease progression?
Combining bevacizumab with different chemotherapy regimens
It is not known whether the benefits of bevacizumab are restricted to the treatment regimen used in E4599. I don’t believe one standard therapy exists; there are probably three to five reasonable platinum-based doublets. However, we should evaluate safety data from Phase I/II trials before combining bevacizumab with other regimens.
Another issue is determining which treatment to utilize in patients progressing after first-line platinum-based therapy. Typically, in the second-line setting, my cytotoxic drug of choice is pemetrexed. However, it is unknown if bevacizumab should be combined with pemetrexed or if bevacizumab will work as well in the second-line setting or beyond. Ongoing exploratory, randomized Phase II trials in the second-line setting are evaluating the use of bevacizumab with pemetrexed. The magnitude of benefit is difficult to estimate, but I believe the benefit of bevacizumab in the second-line setting will be similar to that observed in the first-line setting.
Most likely, docetaxel and bevacizumab could be combined safely, but additional data are needed to ensure that the toxicity profile of docetaxel is not dramatically different from that of paclitaxel when used in combination. Considering the safety and efficacy data from E4599, switching from carboplatin plus docetaxel to carboplatin plus paclitaxel is an option.
Selection of chemotherapy in patients with metastatic disease and a contraindication to anti-VEGF therapy
In patients with metastatic disease who do not meet the eligibility criteria for ECOG-E4599, my approach has been to utilize a platinum-based therapy for four cycles. Depending on a patient’s concerns about toxicity, I treat half of my patients with carboplatin and a taxane and the other half with carboplatin and gemcitabine in the first-line setting.
The Coalition trial (Treat 2005) was the first head-to-head comparison of carboplatin/paclitaxel versus carboplatin/gemcitabine. The survival and response outcomes were similar between those two regimens, but the toxicity profiles were different. Carboplatin/gemcitabine was associated with more bone marrow toxicity (anemia, neutropenia and thrombocytopenia), while carboplatin/paclitaxel had more nonhematologic toxicity, particularly neuropathy and alopecia.
I’ve been using carboplatin/paclitaxel for a long time, and that’s typically what I use outside of a clinical trial. My use of carboplatin/docetaxel has mostly been restricted to use in clinical trials, but I believe carboplatin/docetaxel is a reasonable regimen.
Role of adjuvant erlotinib in patients with an EGFR mutation
In Stage III disease, a compelling argument can be made to use erlotinib as adjuvant therapy following chemotherapy in patients with known genetic mutations. However, the duration of therapy, the duration of response and the true magnitude of benefit are unknown in this setting. Although select patients, such as those with genetic mutations, are more sensitive to oral tyrosine kinase inhibitors, not all patients with genetic mutations are sensitive to these agents. Due to the lack of data, one must make the best clinical judgment.
I would not administer erlotinib alone as adjuvant therapy in a patient with a known genetic mutation, as the standard is platinum-based doublet therapy for three or four cycles. However, based on current data, I would talk to the patient about the potential benefits of receiving sequential erlotinib. Because most patients who relapse after lung cancer surgery do so within two to three years, I would probably limit treatment to three years, when the patient is at the highest risk for disease recurrence.

ANITA trial
The Adjuvant Navelbine International Trialist Association (ANITA) results presented at the 2005 ASCO meeting were similar to those of the NCIC CTG BR10 study and confirmed the survival data presented by the Canadian group at ASCO 2004 and data from CALGB-9633, which showed a clear survival benefit associated with adjuvant treatment (Douillard 2005; [3.3]). Both ANITA and BR10 studied the same regimen, and the magnitude of benefit was roughly the same. The ANITA trial included patients with Stage IB through IIIA disease, whereas the Canadian study did not include patients with Stage IIIA disease.
ANITA is the fourth straight large adjuvant trial to show a clear survival benefit in resected NSCLC. I believe the book is closed on this, and we can begin debating the optimal adjuvant strategy.
Chemoradiation versus chemoradiotherapy followed by surgery
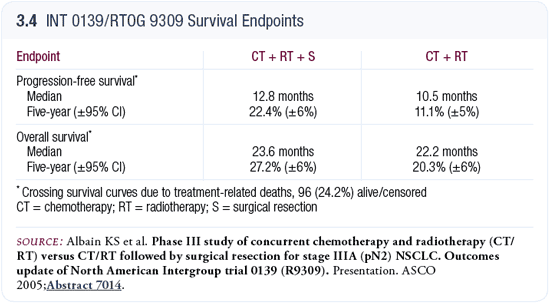
At ASCO, Kathy Albain updated the Intergroup trial 0139 evaluating chemoradiotherapy versus chemoradiotherapy followed by surgery (Albain 2005; [3.4]). The bottom line from that trial is not different than it was a year or two ago: Survival is the same on both arms. An advantage in progression-free survival seems to exist for the surgical arm, but that does not translate into a survival advantage.
The one observation they’ve refined somewhat is the risk that patients who require a pneumonectomy are undertaking. The “punch line” from that trial is that if a pneumonectomy is required, surgery is probably not the right thing to do in this population.
The subsequent trial that will be done by the Intergroup will take a more select group of patients, probably those with less bulky IIIA disease and good staging (3.5). The trial will evaluate chemotherapy followed by surgery versus chemotherapy/ radiation induction followed by surgery. On the chemotherapy-alone arm, it will be cisplatin and docetaxel for two or three cycles, while on the chemoradiotherapy arm, they will use cisplatin and docetaxel at attenuated doses in order to administer it with radiation therapy. After surgery, patients are supposed to receive three cycles of docetaxel.

Select publications
 |
Dr Socinski is an Associate Professor of Medicine in the Multidisciplinary Thoracic Oncology Program at the University of North Carolina’s Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina. |
|

