You are here: Home: LCU 4 | 2005 : Vincent A Miller, MD
Vincent A Miller, MD |
EDITED COMMENTS |
 ECOG-E4599: Carboplatin/paclitaxel with or without bevacizumab in patients with previously untreated advanced nonsquamous NSCLC ECOG-E4599: Carboplatin/paclitaxel with or without bevacizumab in patients with previously untreated advanced nonsquamous NSCLC
This study was a major stepping stone in the therapy for patients with NSCLC. We’ve never had a three-drug combination beat a two-drug combination.
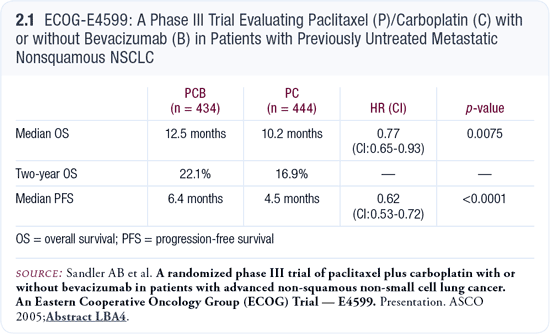
In large US cooperative group studies of patients with Stage IV disease, we’ve also never had a median survival greater than one year. In ECOG-E4599, at two years, we now have survival of about 20 percent of the patients treated with carboplatin/paclitaxel and bevacizumab (Sandler 2005; [2.1]).
This has been “soft pedaled” or felt to be a modest benefit by some, but for those of us who have worked in the field for a while, this is important proof of principle that we can do better for our patients. E1594 was described as the plateau trial, with the belief that we couldn’t obtain a median survival beyond eight months (Schiller 2002).
Interestingly, patients in the carboplatin/paclitaxel arm of E4599 did better than those in E1594. Perhaps some patients are doing better with our second- and third-line therapies. Pemetrexed, gefitinib and erlotinib were available to some patients on this study.
Even though these drugs may not be a home run, they are a series of walks, infield hits and singles. We’re scoring points and having people live longer with this disease.
We all wanted to see the data to make sure there were no unusual patterns predictive of toxicity or groups that were particularly sensitive or insensitive to the combination, and we have been reassured that the safety profile is robust. The drug may have different toxicities than cytotoxic agents, but that doesn’t mean it has more or worse toxicities. Overall, it’s a tolerable regimen. We are interested in using bevacizumab in our patients who fit the profile of the patients treated in E4599.*
* Note: Nonsquamous cell Stage IIIB or IV NSCLC; no history of hemoptysis; no CNS metastasis.
Phase I/II trial of erlotinib and bevacizumab in patients with recurrent Stage IIIB/IV nonsquamous NSCLC
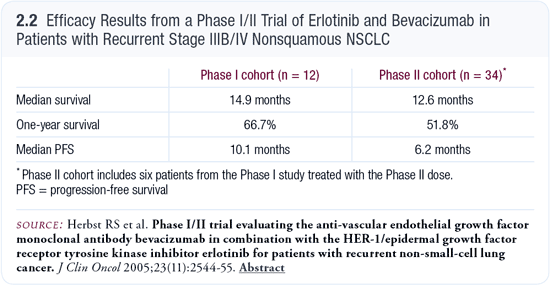
In this trial, it was nice to see that the drugs were safe to administer together at the full doses (150 mg daily for erlotinib and 15 mg/kg every three weeks for bevacizumab). We saw clear activity of the combination (Herbst 2005; [2.2]). Another trial will compare bevacizumab plus erlotinib to erlotinib alone in patients with adenocarcinomas. Among patients with adenocarcinomas, two obvious sets of patients exist. Patients who have a negligible or no smoking history are likely to have EGFR gene mutations. Those patients will benefit greatly from erlotinib and may or may not need bevacizumab. At the other end are patients who are heavy smokers in whom erlotinib may not be helpful and bevacizumab may be a key drug. Our task is to characterize them by a testing or stratification process so we can deliver the best therapies on a patient-specific basis.
First-line therapy for patients with metastatic NSCLC and an EGFR gene mutation
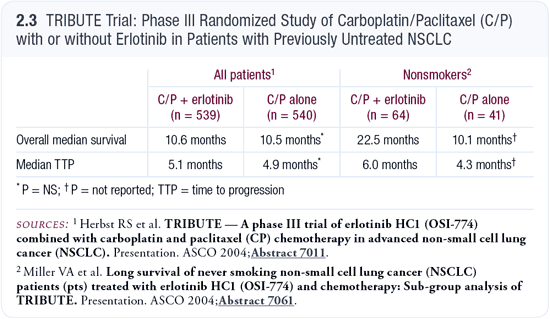
We’re big believers in the positive predictive value of EGFR gene mutations, particularly in exons 19 and 21. We are also privileged to have the EGFR gene mutation test available at Memorial Sloan-Kettering. If I have a patient with an EGFR gene mutation, I treat them initially with chemotherapy and erlotinib, based on the subgroup analysis of the never-smokers from the TRIBUTE trial (Miller 2004; [2.3]). We don’t have much data on mutations from large series of patients in terms of response and whether chemotherapy is necessary, but my intuition is that it’s a reasonable course of action.
I’m not sure my approach to these patients would change because of the results of ECOG-E4599. You could, however, envision bevacizumab/erlotinib or the four drugs (carboplatin/paclitaxel/bevacizumab/ erlotinib) as first-line therapy in that subset of patients. A number of different scenarios exist. In that modestsized subset, I would probably use carboplatin/paclitaxel and erlotinib. For the patients who aren’t likely to benefit from erlotinib, I would use bevacizumab.
Relationship between smoking history and EGFR gene mutation
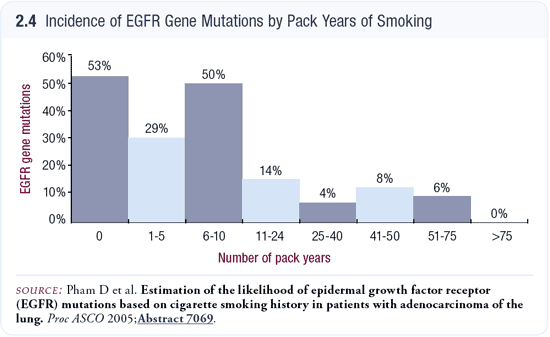
We evaluated patients who had smoking histories of varying degrees to determine the frequency of EGFR gene mutations. We arbitrarily divided patients according to units of pack years. Patients with a smoking history of 10 pack years or less, particularly those who had quit many years ago (≥25 years ago), had a likelihood of having an EGFR gene mutation that was as high as the individual who never smoked (Pham 2005; [2.4]). This observation helps the clinician who doesn’t have access to the EGFR gene mutation test or doesn’t want to wait three weeks for the results. If you believe in the EGFR gene mutation like we do, it’s a guiding feature that helps in clinical decision-making.
Adjuvant therapy for patients with EGFR gene mutations
In certain adjuvant therapies, it is more important to know if the EGFR gene mutations are predictive. If a therapy works 50 or 70 percent of the time in the metastatic setting in patients with the EGFR gene mutation, it will be that much more efficacious in locoregional disease. If I were a patient with an EGFR gene mutation who had a surgical resection, I’d be running to take erlotinib.
I would use one of two approaches: (1) chemotherapy alone for four cycles, followed by erlotinib for a few years or (2) erlotinib and chemotherapy concomitantly from the beginning, as in the TRIBUTE trial in patients with metastatic disease. Either approach would be acceptable. In my most recent patients, I’ve used chemotherapy alone first, followed by erlotinib.
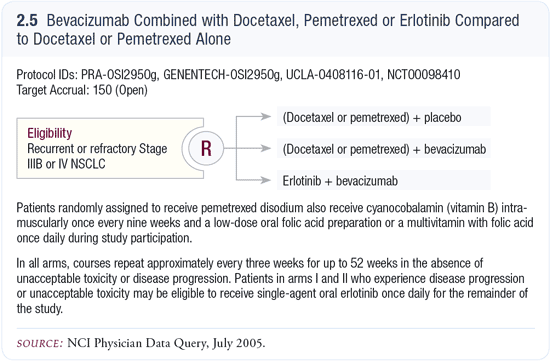
Incorporation of bevacizumab with docetaxel or pemetrexed
In an important ongoing study, patients are randomly assigned to one of three arms: (1) placebo plus either docetaxel or pemetrexed (according to the physician’s choice), (2) bevacizumab plus either docetaxel or pemetrexed or (3) bevacizumab plus erlotinib (2.5). That’s an important question that speaks to the potential use of bevacizumab in the second-line setting.
For example, if you saw a much higher response rate in patients who received pemetrexed with bevacizumab versus pemetrexed alone, given how powerful bevacizumab was in the first-line setting, that would indicate that this approach is reasonable. If there were no safety and insurance issues, I would certainly consider that doublet in the second-line setting.
Select publications
 |
Dr Miller is an Associate Attending Physician of Thoracic Oncology Service at Memorial Sloan-Kettering Cancer Center in New York, New York.
|
|

