You are here: Home: LCU 4 | 2005 : Roman Perez-Soler, MD
Roman Perez-Soler, MD |
EDITED COMMENTS |
 Hemorrhagic complications in E4599 Hemorrhagic complications in E4599
The big news from ASCO this year is that in ECOG-E4599, bevacizumab prolongs median survival by about two months when added to carboplatin/paclitaxel (Sandler 2005). Attempts to create a triplet drug combination have been a big frustration in thoracic oncology for years.
These are modest improvements, but they are a step up. Based on this information, people will and should use the combination. The main concern, which might be overstated, is bleeding.
However, in E4599, the numbers were four percent for bleeding and one percent for death related to bleeding (Sandler 2005). These numbers are small, so I’m not too concerned.
From time to time in the clinic, we have patients who die by exsanguination. It’s rare, but it happens, and we don’t know if the bleeding in E4599 is a manifestation of tumor response. It probably is because it tends to occur more in tumors that are necrotic. By disturbing the vascularity in those tumors, they bleed more. I cannot tell you that spontaneous bleeding occurs more often in patients with squamous cell cancer; however, it certainly happens more often in patients with central tumors — those close to the bronchus — than when a tumor is in the parenchyma, because the parenchyma contains the bleeding.
We will learn how to handle the toxicity associated with bevacizumab. I’m driven more by efficacy, and the toxicity is manageable. Obviously, we should also be evaluating — and we’ve started seeing data — which groups of patients may benefit the most.
From the analysis, it seems that males derive more benefit than females, which is intriguing (Sandler 2005). I don’t think it will turn out to be true because in patients with breast cancer, bevacizumab works well.
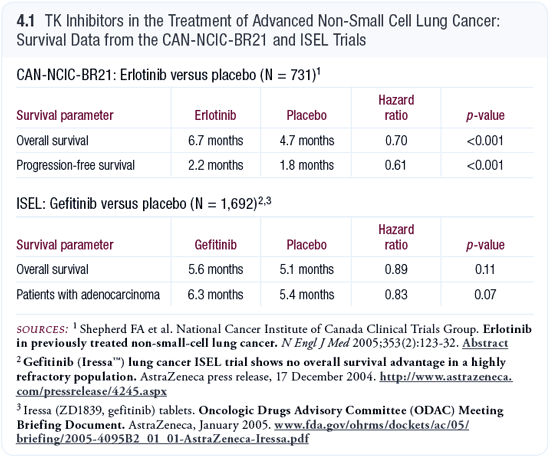
Comparing the ISEL and CAN-NCIC-BR21 trials
I believe gefitinib is underdosed, and that’s probably why the ISEL trial failed (4.1). The incidence of rash was 35 percent (Carroll 2004) in the ISEL trial and 75 percent in CAN-NCIC-BR21 (Shepherd 2004). For the extremely sensitive mutated tumor that’s going to respond, dose doesn’t make a difference. But for the older male patient with squamous cell carcinoma and a poor performance status, the tumor is sensitive to dose. Those are the patients in whom you must maximize the dose to block as many of the receptors as possible.
Adjuvant therapy for nonsmokers or patients with EGFR gene mutations
I would bring up the issue of adjuvant erlotinib with these patients. I tend to be liberal in terms of how to use therapies. Patients have the right to make choices, not just based on randomized trials but rather on emerging evidence. If I were a patient, I would pick the latest treatment that looks promising. I don’t need a randomized study to accept a therapy, particularly if I know the risk is low. Obviously, if a risk of death existed, I would think twice. So I would discuss adjuvant erlotinib with any patient who is young with children, female, a nonsmoker and has adenocarcinoma that is being resected.
I would explain to the patient that adjuvant chemotherapy has been shown to work, and I would do an EGFR gene mutation analysis. If it were positive, maintenance erlotinib would be reasonable after adjuvant chemotherapy, based on our current knowledge. I would probably use maintenance erlotinib for six months or one year. It would be completely intuitive medicine, and you cannot practice that way unless the patient understands exactly what you are doing. If the patient does not and is skeptical, I wouldn’t push. If I’m not comfortable that the patient understands, I would use the standard of care.
Predictors of response and survival with erlotinib
In CAN-NCIC-BR21, out of roughly 700 patients, one third received placebo and two thirds received erlotinib. Dr Tsao presented data at ASCO 2005 from approximately 160 samples obtained from that study. They found that approximately 20 percent of the samples had EGFR gene mutations. However, half of those mutations were the ones reported by the Harvard group, and the other half were new ones that no one has described (Tsao 2005). We don’t know if they were sensitizing mutations or if it might have been the result of technological problems. The frequency of the mutations was more or less what we would have expected, but half of them were strange mutations.
Based on the data from the Harvard and Memorial groups, you would expect at least a 60 percent response rate for the patients with the EGFR gene mutation, and the response rate was 16 percent (Tsao 2005). The study also evaluated the survival of patients with the mutated EGFR gene, and erlotinib had no impact on survival in those patients (Tsao 2005). That’s the bad news, which leaves us with the question, should we send tumors for mutation testing? Maybe not. If the patient has all the clinical characteristics, you already have a sense of the probability of response.
In the same study, the amplification of the EGFR gene by FISH was evaluated. They found clearly that if the patient had an amplified EGFR gene, the chance of response and survival was much better. The hazard ratio for survival was 0.4 for the patients with amplified EGFR genes. That was as strong as the results for the nonsmoking patients in that trial. Nonsmoking status was number one clinically, and now EGFR gene amplification is number one pathologically — both with a 0.4 hazard ratio (Tsao 2005). No data were reported on nonsmokers with EGFR gene amplification. That hazard ratio may be even lower.
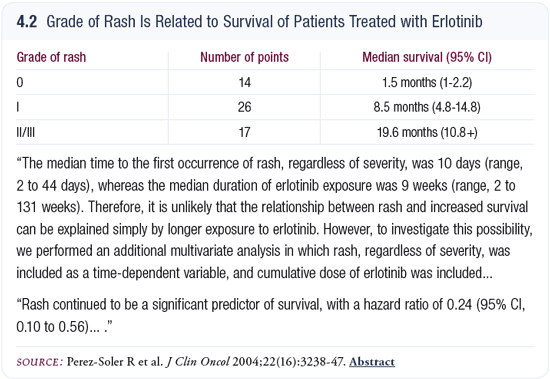
Rash and response to the EGFR tyrosine kinase inhibitors
The lack of rash at 30 days is another negative predictor of response to the EGFR tyrosine kinase inhibitors. I have always been “the rash person.” I’m a strong believer that rash is correlated with survival. If a patient doesn’t develop a rash after 30 days of erlotinib at full doses, the curves show their median survival is about two months (Perez-Soler 2004; [4.2]).
The lack of rash always indicates you’ve selected a group of patients who do poorly. If I don’t see any rash at 30 days, I use a higher dose. I start with a dose of 150 mg of erlotinib and at one month, if the patient does not have a rash or diarrhea, I escalate the dose to 200 mg. I’ve done it up to 250 mg. If I don’t see any rash with a higher dose, I start to think I’m wasting time.
Select publications
 |
Dr Perez-Soler is the Chairman of the Department of Oncology at Montefiore Medical Center and Gutman Professor of Medicine at Albert Einstein College of Medicine in Bronx, New York. |
|

