|
You are here: Home: LCU 4 | 2005 : Editor's Note
 |
|
 |
| Editor’s Note |
 |
|
| Disease within a disease |
“Breast cancer is more than one disease, and HER2-positive breast cancer may be as different from HER2-negative breast cancer as acute myeloid leukemia is from acute lymphocytic leukemia or as different as pneumococcal pneumonia is from staphylococcal pneumonia. We finally have the tools to show us those differences, and even more exciting, we have therapies to take advantage of those differences. I predict that in a couple of years, we’re going to have to rethink all of the major conceptual paradigms that we use right now to treat breast cancer.”
— Harold J Burstein, MD, Breast Cancer Update Audio Series |
Hal Burstein’s prediction about breast cancer came true on the afternoon of May 16th in Orlando during the ASCO meeting with a succession of four stunning presentations on the effects of adjuvant trastuzumab in major Phase III randomized trials. In an instant, clinical practice had undergone a revolution for the approximately 40,000 to 50,000 patients with HER2-positive breast cancer. From a research perspective, a new model of targeted treatment had achieved its pinnacle. An unspoken undercurrent was that Hal Burstein’s vision of a “disease within a disease” had come to pass. Not only will day-to-day care for these patients be altered radically, but current and future clinical trials are now also being reconsidered.
For example, ongoing randomized studies of various forms of chemotherapy — such as NSABP-B-38 and SWOG-S0221 — have now essentially become studies for patients with HER2-negative breast cancer because patients with HER2-positive disease will undoubtedly receive adjuvant trastuzumab and thus will not be eligible. What about adjuvant endocrine trials? Will they need to be altered to allow trastuzumab for ER-positive, HER2-positive cases? In the three adjuvant trastuzumab trials presented on May 16th, about half of the patients had ER-positive, HER2-positive tumors.
Sometimes it seems like breast cancer is the wise grandmother who serves as a role model to other less developed oncologic clinical research cultures. Our CME group has uncovered repeated instances in which it has been helpful to make comparisons between breast cancer and other tumors. At the moment, the best analogy I see to the HER2/trastuzumab story in solid tumor oncology relates to non-small cell lung cancer — specifically, the 10 to 20 percent of the NSCLC population that can be differentiated by a high rate of tumor response to tyrosine kinase inhibitors. Let’s call these tumors TKIR (tyrosine kinase inhibitor responsive). From what I can gather in chatting with pulmonary oncology mavens interviewed for this series, it seems that these lung cancer tumors respond to TKIs at least as robustly (favorite new word for researchers replacing “signal”) as HER2-positive breast cancer responds to trastuzumab in the metastatic setting (eg, Chuck Vogel’s key trial demonstrating a 35 percent objective tumor response rate in patients with FISH-positive tumors).
Here’s the problem and concern: It was not until many years after studies in the metastatic setting that clinical trials proved adjuvant trastuzumab was relatively safe and added an impressive level of tumor control combined with or following chemotherapy. With perhaps 15,000 people a year dying of TKIR NSCLC, I don’t much like the idea of waiting that long for an answer. Perhaps the most significant challenge facing investigators leading the next wave of trials focusing on the TKIRs is figuring out who fits into this important group. From what I could ascertain at ASCO by attending sessions and speaking with the three interviewees on this program, the first step is to focus on nonsmokers and perhaps former smokers who have less than a yet-to-be-defined amount of tobacco exposure — perhaps 10 pack years.
Additional phenotypic qualities of the TKIR group are female gender, Asian background and adenocarcinoma histology, particularly with BAC features. Of course, another key identifying factor is the presence of an EGFR mutation in the tumor. However, I left ASCO believing that quality control with the assay is lacking and the mutation’s correlation with response to TKIs is far from a given. FISH and IHC assays of EGFR may turn out to be better predictors. Future clinical trials focusing on TKIR NSCLC might have smoking history as a primary eligibility factor. Other patients might enter based on documented gene mutations and phenotypic factors. The question is: What kind of adjuvant trial design makes sense?
In that regard, another major card tossed on the table at ASCO was the anti- VEGF agent bevacizumab. As discussed on the last issue of Lung Cancer Update by principal investigator Alan Sandler, ECOG trial E4599 — presented by Dr Sandler at a major plenary session — demonstrated for the first time in NSCLC that the use of three systemic agents as first-line therapy resulted in greater progression-free and overall survival than two agents. In this case, the third partner was bevacizumab, which provided a clinically meaningful improvement in tumor control with a minimal increase in side effects and toxicity when combined with paclitaxel-carboplatin in carefully selected patients (eligibility criteria: Stage IIIB, IV or recurrent nonsquamous NSCLC with a PS of 0-1).
As with colorectal cancer and breast cancer, bevacizumab now deserves to be rapidly studied in the adjuvant setting, and it will be interesting to see if new adjuvant trials in lung cancer divide patients into TKIR and non-TKIR subsets. In this regard, my “dream trial” (1.1) would have six randomization arms — some of which would undoubtedly be squashed by CTEP, the FDA and other powers that be. If this type of trial has merit, a major issue would be accrual, specifically related to the question of whether oncologists and patients would be comfortable with the two arms not containing erlotinib. If I were a nonsmoker with NSCLC or had a tumor with an EGFR mutation, this would give me cause for great concern, but maybe others will feel differently. Whatever is decided, I sure as hell want to see these studies get done quickly. This disease is devastating thousands of lives a year, but the rapid progression rate and high mortality also means that answers could come sooner, for example, than in breast and colon cancer.
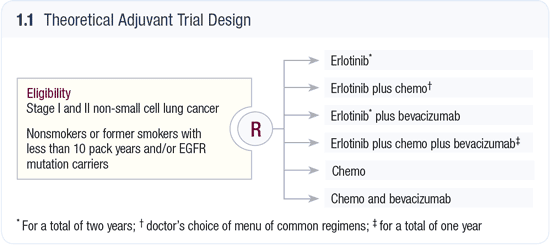
To focus on 10 percent of patients in a disease that decimates the lives of 160,000 people annually may seem questionable, but the mortality in patients with TKIR tumors exceeds that of other important cancers such as gastric cancer, soft tissue sarcoma and melanoma. With trastuzumab, it took many years to find an answer, but my take is that now we know how to execute adjuvant trials more effectively, and maybe that time frame can be cut in half.
As long as I am rambling on about things that maybe I don’t know enough about, here’s another suggestion: Get the NSABP involved. Call it the NSABLP (National Surgical Breast and Bowel and Lung Project). Yes, I know that Norm Wolmark and colleagues have their hands full, but those guys know how to get surgeons on board. They did it in breast, colon, and rectal cancer, and when the casualties are this brutal, we should pull out all the stops.
It is well documented that many or most trial participants join studies primarily to help future patients, although we also know that there is often a direct benefit to those in the trials (witness the participants in the trastuzumab studies who avoided relapse and death via participation). I don’t see any reason why lung cancer patients would be less interested in making a contribution to the greater good than breast cancer patients. Let’s give them that chance now, and maybe there will be another May 16th at a future ASCO meeting with more good news — this time in a disease (NSCLC) that perhaps three years ago was considered stuck in the 1980s but now has the opportunity to take a leadership role in the research-to-practice translation of molecular targeted therapy.
— Neil Love, MD
NLove@ResearchToPractice.net
|
|

