
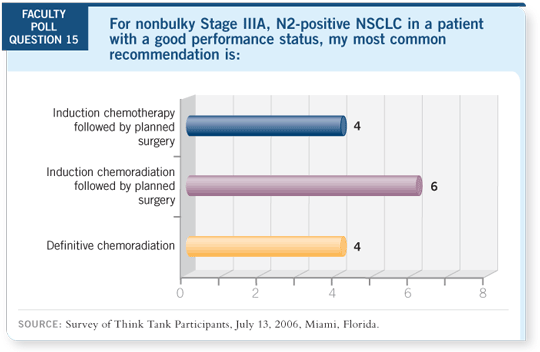
Select Excerpts from the Discussion
CD 2, Track 14, 17
 DR LOVE: Harvey, your RTOG-0412 trial will evaluate induction
cisplatin/docetaxel with or without radiation therapy followed by surgery
and consolidation docetaxel in patients with Stage IIIA NSCLC. Based
on Kathy Albain’s 2005 ASCO report of the RTOG-9309 study, will the
RTOG-0412 study be amended to exclude patients receiving pneumonectomies?
DR LOVE: Harvey, your RTOG-0412 trial will evaluate induction
cisplatin/docetaxel with or without radiation therapy followed by surgery
and consolidation docetaxel in patients with Stage IIIA NSCLC. Based
on Kathy Albain’s 2005 ASCO report of the RTOG-9309 study, will the
RTOG-0412 study be amended to exclude patients receiving pneumonectomies?
 DR PASS: It is difficult to predict which patient is going to require a pneumonectomy
based on the preoperative studies. I am concerned about the right
pneumonectomies, but the data with regard to these sort of morbidities simply
do not bear out at other institutions.
DR PASS: It is difficult to predict which patient is going to require a pneumonectomy
based on the preoperative studies. I am concerned about the right
pneumonectomies, but the data with regard to these sort of morbidities simply
do not bear out at other institutions.
 DR CHOY: We hope this study will change patterns of practice. If you survey
oncologists about whether they use preoperative chemotherapy or chemoradiation
therapy in patients with Stage IIIA disease, you will see an even split. We need to answer the question, and this study will answer it. This is probably the
last time we’ll have this kind of trial.
DR CHOY: We hope this study will change patterns of practice. If you survey
oncologists about whether they use preoperative chemotherapy or chemoradiation
therapy in patients with Stage IIIA disease, you will see an even split. We need to answer the question, and this study will answer it. This is probably the
last time we’ll have this kind of trial.
 DR KRIS: I’m not a big fan of this trial.
DR KRIS: I’m not a big fan of this trial.
 DR LOVE: Mark, which question would you like to see addressed in this
patient population?
DR LOVE: Mark, which question would you like to see addressed in this
patient population?
 DR KRIS: I’d like to compare groups of patients, one of which is receiving
an intervention for which we have some literature-based expectation to
improve survival. That does not exist for the addition of radiation therapy to
chemotherapy as induction.
DR KRIS: I’d like to compare groups of patients, one of which is receiving
an intervention for which we have some literature-based expectation to
improve survival. That does not exist for the addition of radiation therapy to
chemotherapy as induction.
 DR LOVE: What would you like to see studied in this patient population?
DR LOVE: What would you like to see studied in this patient population?
 DR KRIS: Induction erlotinib in people who don’t smoke.
DR KRIS: Induction erlotinib in people who don’t smoke.
 DR LYNCH: Mark, this is still a question that, for 15 years, we’ve danced
around. I believe this question has prevented us from introducing novel agents
for patients with Stage III disease.
DR LYNCH: Mark, this is still a question that, for 15 years, we’ve danced
around. I believe this question has prevented us from introducing novel agents
for patients with Stage III disease.
I would love to avoid the burden of having to use chemoradiation before
surgery. But my radiation oncologist points out that the best data still are with
chemoradiation followed by surgery.
CD 2, Track 24
 DR LOVE: Jack, can you comment on the new data presented at ASCO on
the SWOG-S9504 regimen?
DR LOVE: Jack, can you comment on the new data presented at ASCO on
the SWOG-S9504 regimen?
 DR WEST: The Hoosier Oncology Group trial (HOG LUN 01-24/USO 02-
33) asked the question of whether consolidation docetaxel added anything
to definitive chemoradiation therapy. This trial has been ongoing for a few
years, and the safety data were presented at ASCO 2006. Of the 241 patients
accrued, two thirds were randomly assigned after definitive chemoradiation
therapy to consolidation docetaxel or observation.
DR WEST: The Hoosier Oncology Group trial (HOG LUN 01-24/USO 02-
33) asked the question of whether consolidation docetaxel added anything
to definitive chemoradiation therapy. This trial has been ongoing for a few
years, and the safety data were presented at ASCO 2006. Of the 241 patients
accrued, two thirds were randomly assigned after definitive chemoradiation
therapy to consolidation docetaxel or observation.
Of the patients assigned to docetaxel, only 29 percent were able to complete
three cycles, 22 percent required dose reductions, one third required growth
factor support and five percent required blood transfusions.
The Hoosier Oncology Group presentation highlighted the toxicity challenges.
Of the patients assigned to consolidation docetaxel, 20 percent were hospitalized:
one third for febrile neutropenia, 19 percent for infections without
neutropenia, and 9.5 percent for pneumonitis.
Four treatment-related deaths occurred, accounting for 5.5 percent of the
patients treated with docetaxel (Bedano 2006).
CD 2, Tracks 26-27
 DR LOVE: Rogerio, do you use consolidation docetaxel off study? Do you
use growth factors?
DR LOVE: Rogerio, do you use consolidation docetaxel off study? Do you
use growth factors?
 DR LILENBAUM: Yes to both those questions. The HOG trial is probably not
sufficiently powered to detect a statistically significant difference in outcome
for maintenance docetaxel.
DR LILENBAUM: Yes to both those questions. The HOG trial is probably not
sufficiently powered to detect a statistically significant difference in outcome
for maintenance docetaxel.
 DR LOVE: If it were sufficiently powered, what do you think it would show?
DR LOVE: If it were sufficiently powered, what do you think it would show?
 DR LILENBAUM: I believe it would show a positive result.
DR LILENBAUM: I believe it would show a positive result.
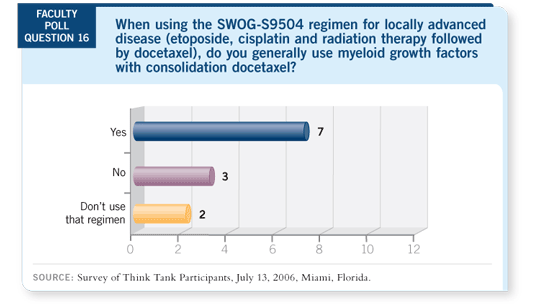
 DR KIM: I like using the SWOG-S9504 regimen. Sometimes it’s difficult to
administer the consolidation therapy. It mostly depends on how the concurrent
chemoradiation is tolerated by the patient.
DR KIM: I like using the SWOG-S9504 regimen. Sometimes it’s difficult to
administer the consolidation therapy. It mostly depends on how the concurrent
chemoradiation is tolerated by the patient.
Hak, when is it safe to use myeloid growth factors around the setting of radiation?
We’re hesitant to use them during radiation, but should we wait six or 10
weeks? Or is it okay to start after the radiation machine is turned off ?
 DR CHOY: I believe this hesitation is because of the old Paul Bunn study using
GM-CSF for patients with small-cell lung cancer who received chemoradiation
therapy. They had significant pneumonitis (Bunn 1995).
DR CHOY: I believe this hesitation is because of the old Paul Bunn study using
GM-CSF for patients with small-cell lung cancer who received chemoradiation
therapy. They had significant pneumonitis (Bunn 1995).
Those are the only data we have at this point, so a lot of people are reluctant
to use growth factors with radiation. I believe you can use them with radiation,
but we have no data. Rogerio is going to conduct an RTOG study of
chemoradiation therapy with G-CSF followed by pegfilgrastim.
 DR LILENBAUM: We took the SWOG-S9504 regimen and added growth
factors during the chemotherapy and radiation therapy, which has not been
done since the Bunn study.
DR LILENBAUM: We took the SWOG-S9504 regimen and added growth
factors during the chemotherapy and radiation therapy, which has not been
done since the Bunn study.
Among the first 10 patients, we’ve had no major complications. We felt it was
reasonable to bring this question into a large Phase II trial. It may change the
way we use chemotherapy and radiation.
Select publications

