
Select Excerpts from the Discussion
CD 1, Tracks 2, 4
 DR LOVE:
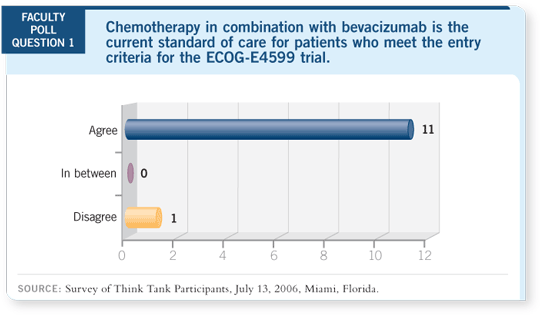
DR LOVE: Tom, do you agree or disagree with the statement that for
a patient who would have met the entry criteria for ECOG-E4599, the
current standard of care is chemotherapy with bevacizumab?
 DR LYNCH: Agree.
DR LYNCH: Agree.
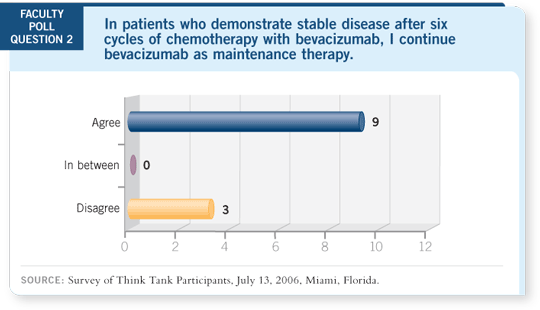
 DR LOVE: Should bevacizumab be continued until disease progression?
DR LOVE: Should bevacizumab be continued until disease progression?
 DR LYNCH: Agree.
DR LYNCH: Agree.
 DR LOVE: Why do you feel that way?
DR LOVE: Why do you feel that way?
 DR LYNCH: If I’m going to practice evidence-based medicine, I’d want to treat
my patients the way they were treated on ECOG-E4599 (Sandler 2005), until we
have evidence that dictates otherwise. I also like the idea of continuing bevacizumab
after finishing the chemotherapy because micrometastatic disease or
smaller-volume disease might be present that you may be influencing.
DR LYNCH: If I’m going to practice evidence-based medicine, I’d want to treat
my patients the way they were treated on ECOG-E4599 (Sandler 2005), until we
have evidence that dictates otherwise. I also like the idea of continuing bevacizumab
after finishing the chemotherapy because micrometastatic disease or
smaller-volume disease might be present that you may be influencing.
 DR LOVE: I’ll ask Alan the same questions.
DR LOVE: I’ll ask Alan the same questions.
 DR SANDLER: The answers are yes and yes. It’s interesting that other clinical investigators
don’t necessarily have the same thoughts. The objections raised include
the fact that this is only one randomized Phase III study, and we’re waiting for a
second. That is fair, and I usually reply, “Is anybody using erlotinib?”
DR SANDLER: The answers are yes and yes. It’s interesting that other clinical investigators
don’t necessarily have the same thoughts. The objections raised include
the fact that this is only one randomized Phase III study, and we’re waiting for a
second. That is fair, and I usually reply, “Is anybody using erlotinib?”
Only one randomized Phase III study supports erlotinib (Shepherd 2005), and
everybody has pretty much jumped on that one. I personally wouldn’t want to
put a family member on a study that included a control arm without bevacizumab.
More toxicity is associated with bevacizumab and chemotherapy than
with chemotherapy alone, but I believe it’s clinically acceptable.
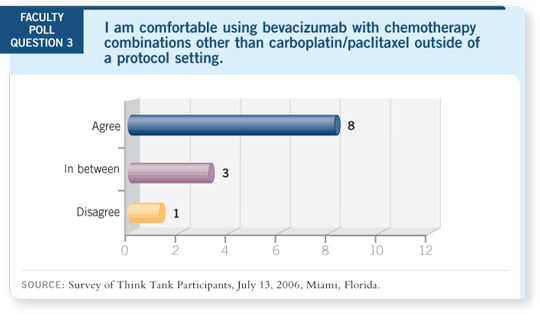
 DR LOVE: What about bevacizumab and other chemotherapy agents?
DR LOVE: What about bevacizumab and other chemotherapy agents?
 DR LYNCH: I need to see data to suggest that you can safely administer
bevacizumab with carboplatin/docetaxel and carboplatin/gemcitabine, but I
suspect you can.
DR LYNCH: I need to see data to suggest that you can safely administer
bevacizumab with carboplatin/docetaxel and carboplatin/gemcitabine, but I
suspect you can.
 DR LOVE: Does “suspect” mean you’re comfortable with using it off protocol?
DR LOVE: Does “suspect” mean you’re comfortable with using it off protocol?
 DR LYNCH: Last night, I was driving to the airport with a practicing oncologist
who’s going to use carboplatin/docetaxel with bevacizumab. He said he has
administered it to patients and had no problems. I expect that it will probably be
okay. However, I won’t use it off protocol until I see some data suggesting it’s safe.
I expect it will be safe, but I’ve stayed with carboplatin/paclitaxel until we have
some of the initial data, which we’ll have very soon.
DR LYNCH: Last night, I was driving to the airport with a practicing oncologist
who’s going to use carboplatin/docetaxel with bevacizumab. He said he has
administered it to patients and had no problems. I expect that it will probably be
okay. However, I won’t use it off protocol until I see some data suggesting it’s safe.
I expect it will be safe, but I’ve stayed with carboplatin/paclitaxel until we have
some of the initial data, which we’ll have very soon.
 DR LOVE: Ed, specifically which chemotherapy regimens are you using with
bevacizumab?
DR LOVE: Ed, specifically which chemotherapy regimens are you using with
bevacizumab?
 DR KIM: At MD Anderson, we’re enrolling patients in a trial of carboplatin/
docetaxel and bevacizumab. We have about 14 patients on the trial, and we haven’t seen many serious toxicities. We had one person with hemoptysis who
had an adenocarcinoma, a noncavitary lesion.
DR KIM: At MD Anderson, we’re enrolling patients in a trial of carboplatin/
docetaxel and bevacizumab. We have about 14 patients on the trial, and we haven’t seen many serious toxicities. We had one person with hemoptysis who
had an adenocarcinoma, a noncavitary lesion.
It wasn’t in the mediastinum, but it was more centrally located. It was an
incidental hemoptysis that occurred, and we took him off the study.
It was interesting because the tumor response was cavitary. We also had one
person who had febrile neutropenia, but you expect some of that with chemotherapy
alone.
 DR LILENBAUM: It’s the gemcitabine-based doublets that remain somewhat
worrisome. I believe most people feel comfortable using docetaxel as opposed
to paclitaxel. Many people have done it safely. I’m not sure why it should be a
concern.
DR LILENBAUM: It’s the gemcitabine-based doublets that remain somewhat
worrisome. I believe most people feel comfortable using docetaxel as opposed
to paclitaxel. Many people have done it safely. I’m not sure why it should be a
concern.
Gemcitabine is more of a concern. We are doing a Phase II study of oxaliplatin
and gemcitabine with bevacizumab. We haven’t seen any significant complications,
such as bleeding or thrombocytopenia, or more episodes of febrile
neutropenia than expected. So I expect it to turn out okay. The best evidence
for that is that in an NCI Phase III adjuvant study, a setting in which you want
to be extra careful about toxicities, most people felt comfortable including a
gemcitabine-based regimen.
 DR HERBST: I believe one of the reasons people feel comfortable is that the
registration trial in Europe, evaluating bevacizumab with chemotherapy, is
being conducted with gemcitabine combinations. Although those data are
talked about, we haven’t seen them, but one would assume that people have
considered that database as they’ve gone forward. My feeling is that we can
probably use all the combinations, but I agree that we need to see the data.
DR HERBST: I believe one of the reasons people feel comfortable is that the
registration trial in Europe, evaluating bevacizumab with chemotherapy, is
being conducted with gemcitabine combinations. Although those data are
talked about, we haven’t seen them, but one would assume that people have
considered that database as they’ve gone forward. My feeling is that we can
probably use all the combinations, but I agree that we need to see the data.
CD 1, Track 6
 DR LOVE:
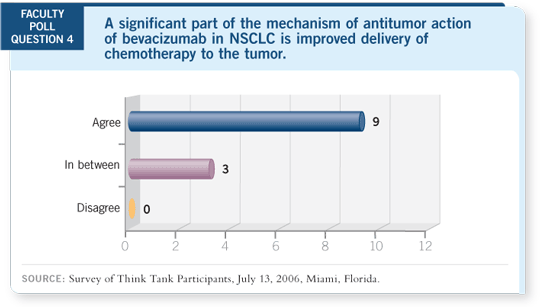
DR LOVE: Roy, can you comment on the mechanism of action of bevacizumab?
 DR HERBST: We really don’t know. Is it working by enhancing chemotherapy?
That’s one thought. Is it working to increase drug delivery? Probably. Is it working
directly on tumor cells? Data suggest that tumor cells have VEGF receptors. Is it
working in the maintenance setting to suppress angiogenesis and endothelial and
tumor cells? Because we have many more compounds coming down the pike, we
need to do some mechanistic studies.
DR HERBST: We really don’t know. Is it working by enhancing chemotherapy?
That’s one thought. Is it working to increase drug delivery? Probably. Is it working
directly on tumor cells? Data suggest that tumor cells have VEGF receptors. Is it
working in the maintenance setting to suppress angiogenesis and endothelial and
tumor cells? Because we have many more compounds coming down the pike, we
need to do some mechanistic studies.
CD 1, Tracks 11-12
 DR LOVE:
DR LOVE: Tom, when you start a patient on chemotherapy and bevacizumab
in the first-line metastatic setting, what do you tell them about the risks?
 DR LYNCH: I tell them that their risk of dying from chemotherapy increases from
about one percent to about three to four percent, but I emphasize that the overall
risk of dying from the disease will be reduced. In the end, the chance of living
longer will be greater on chemotherapy combined with bevacizumab.
DR LYNCH: I tell them that their risk of dying from chemotherapy increases from
about one percent to about three to four percent, but I emphasize that the overall
risk of dying from the disease will be reduced. In the end, the chance of living
longer will be greater on chemotherapy combined with bevacizumab.
We talk about hemoptysis and the fact that it’s rare but can still happen. We
also talk about stroke and other thromboembolic phenomena. When we
consider the colorectal experience with bevacizumab, we’re all pretty confident
that bevacizumab is associated with a real but small increase in thromboembolic
phenomena, which is not trivial for patients with lung cancer.
I believe that in the end, what drives our enthusiasm for bevacizumab is the
fact that in a trial of patients with Stage IV disease, it demonstrated a substantial
and clinically meaningful prolongation of survival (Sandler 2005). This is why I
believe all of us here would endorse using it in this setting. You have to inform
your patient about the risks, and I believe most patients will decide it’s worth it.
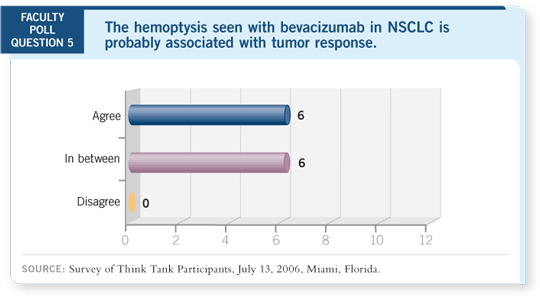
 DR LOVE: It’s been said that perhaps hemoptysis is a manifestation of response.
Can you comment on that?
DR LOVE: It’s been said that perhaps hemoptysis is a manifestation of response.
Can you comment on that?
 DR SANDLER: My bias, particularly for the squamous cell histology, is that
it may be a manifestation of a brisk response to treatment and that cavitation
ultimately results.
DR SANDLER: My bias, particularly for the squamous cell histology, is that
it may be a manifestation of a brisk response to treatment and that cavitation
ultimately results.
CD 1, Track 14
 DR LOVE:
DR LOVE: Roy, can you discuss findings from the trial combining bevacizumab
with erlotinib that was presented at ASCO 2006?
 DR HERBST: A randomized Phase II trial of 120 patients was reported by Lou
Fehrenbacher from Kaiser at ASCO. The treatment arms included chemotherapy
(docetaxel or pemetrexed) with placebo or bevacizumab versus bevacizumab
with erlotinib. In this second-line setting, bevacizumab enhanced both
the EGFR inhibitor, erlotinib, and chemotherapy (Fehrenbacher 2006).
DR HERBST: A randomized Phase II trial of 120 patients was reported by Lou
Fehrenbacher from Kaiser at ASCO. The treatment arms included chemotherapy
(docetaxel or pemetrexed) with placebo or bevacizumab versus bevacizumab
with erlotinib. In this second-line setting, bevacizumab enhanced both
the EGFR inhibitor, erlotinib, and chemotherapy (Fehrenbacher 2006).
The results were reasonably good regarding time to progression, but the
numbers were small. The hazard ratios were 0.66 for chemotherapy in combination
with bevacizumab and 0.72 for erlotinib in combination with bevacizumab.
The 95 percent confidence intervals crossed one. So these were not
statistically significant data, but they were suggestive of a trend. Toxicity and drug discontinuation due to adverse events were much less frequent among
the patients who received the less toxic erlotinib/bevacizumab combination
(Fehrenbacher 2006).
CD 1, Track 17
 DR LOVE:
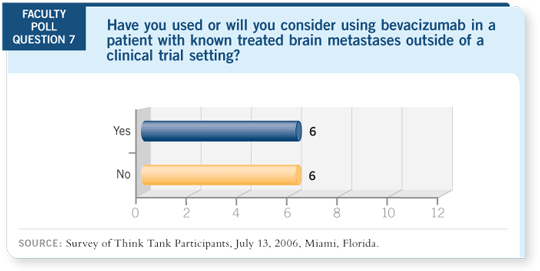
DR LOVE: Outside of a clinical trial, have you used or would you
consider using bevacizumab for a patient with previously treated brain
metastases?
 DR LYNCH: No. We’re participating in the trial to answer that question.
Patients have their brain metastases radiated first, and then they receive treatment.
Because of the restrictions in eligibility for ECOG-E4599, I believe we
have to follow an evidence-based approach, and I have not been using bevacizumab
in this setting outside of a protocol.
DR LYNCH: No. We’re participating in the trial to answer that question.
Patients have their brain metastases radiated first, and then they receive treatment.
Because of the restrictions in eligibility for ECOG-E4599, I believe we
have to follow an evidence-based approach, and I have not been using bevacizumab
in this setting outside of a protocol.
 DR LOVE: What about resected brain metastases?
DR LOVE: What about resected brain metastases?
 DR LYNCH: Resected brain metastases would not have been included in
ECOG-E4599.
DR LYNCH: Resected brain metastases would not have been included in
ECOG-E4599.
 DR MILLER: Aren’t we amending the current clinical trials to allow patients with
previously radiated brain metastases? These contraindications have relative degrees.
Certainly squamous histology and hemoptysis are much more powerful contraindications
(Gordon 2001). This drug is very active in patients with glioblastoma
multiforme — huge tumors with lots of edema — and we’re undertaking approval strategy trials for those patients. We usually obtain the blessing of a neurologist to
use bevacizumab, but we certainly have done it.
DR MILLER: Aren’t we amending the current clinical trials to allow patients with
previously radiated brain metastases? These contraindications have relative degrees.
Certainly squamous histology and hemoptysis are much more powerful contraindications
(Gordon 2001). This drug is very active in patients with glioblastoma
multiforme — huge tumors with lots of edema — and we’re undertaking approval strategy trials for those patients. We usually obtain the blessing of a neurologist to
use bevacizumab, but we certainly have done it.
 DR HERBST: I would wait until the data are available, which I expect will
be soon. One trial, called PASSPORT, will determine if you can use chemotherapy
with bevacizumab for patients with previously treated brain metastases.
DR HERBST: I would wait until the data are available, which I expect will
be soon. One trial, called PASSPORT, will determine if you can use chemotherapy
with bevacizumab for patients with previously treated brain metastases.
Select publications

