
Select Excerpts from the Discussion
CD 1, Tracks 20-22
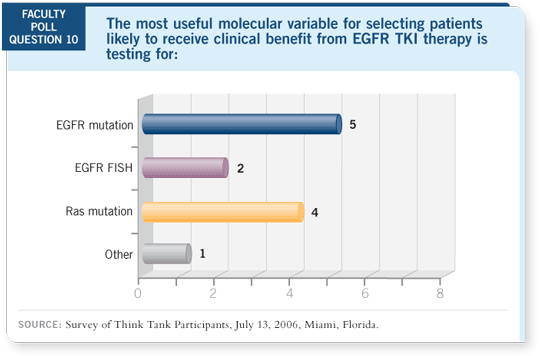
 DR LOVE: Vince, can you discuss the role of EGFR mutation testing in
clinical practice?
DR LOVE: Vince, can you discuss the role of EGFR mutation testing in
clinical practice?
 DR MILLER: Doctors can send slides to Genzyme and obtain a mutation status
within seven to 10 working days with adequate tissue. We know KRAS is an
adverse prognostic factor in lung cancer, and patients with KRAS mutations
probably do not benefit from EGFR tyrosine kinase inhibitors, and they’re
better suited to chemotherapy (Tsao 2006).
DR MILLER: Doctors can send slides to Genzyme and obtain a mutation status
within seven to 10 working days with adequate tissue. We know KRAS is an
adverse prognostic factor in lung cancer, and patients with KRAS mutations
probably do not benefit from EGFR tyrosine kinase inhibitors, and they’re
better suited to chemotherapy (Tsao 2006).
We’re designing a trial at Memorial in which patients will have a biopsy
and we’ll look for the KRAS mutation. We will exclude those patients
with KRAS mutations because we don’t consider it fair for them to receive
erlotinib.
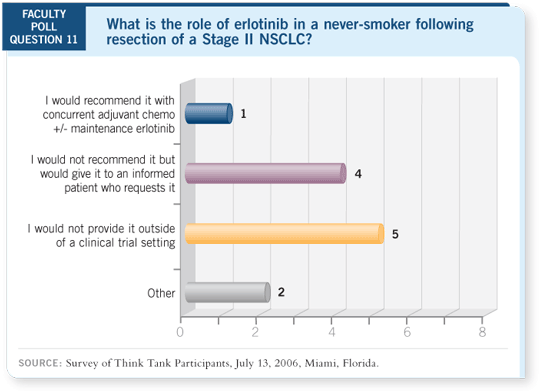
 DR LOVE: From a practical clinical perspective, in which situations —
adjuvant, locally advanced or metastatic — do you think a doctor should
consider using these assays?
DR LOVE: From a practical clinical perspective, in which situations —
adjuvant, locally advanced or metastatic — do you think a doctor should
consider using these assays?
 DR MILLER: There are those who argue that with advanced disease, a one-month
trial with erlotinib can be performed rather than doing the assays, but Rogerio’s
data suggest to me that in an unselected population you might be better off with
chemotherapy (Lilenbaum 2006).
DR MILLER: There are those who argue that with advanced disease, a one-month
trial with erlotinib can be performed rather than doing the assays, but Rogerio’s
data suggest to me that in an unselected population you might be better off with
chemotherapy (Lilenbaum 2006).
Certainly in the adjuvant setting I’d want to know the mutation status because I
would want adjuvant erlotinib if I had an EGFR mutation or was a never smoker.
 DR PASS: Do we have any data on adjuvant erlotinib?
DR PASS: Do we have any data on adjuvant erlotinib?
 DR EDELMAN: There are no data to support the use of an expensive drug
with significant toxicity.
DR EDELMAN: There are no data to support the use of an expensive drug
with significant toxicity.
 DR PASS: In my heart of hearts, for the patient who is a never smoker or
has a mutation, I have to say that I can’t, off trial, dissuade him or her from
erlotinib because it makes sense to me.
DR PASS: In my heart of hearts, for the patient who is a never smoker or
has a mutation, I have to say that I can’t, off trial, dissuade him or her from
erlotinib because it makes sense to me.
Obviously, the trial must be performed so we have the answer to Marty’s
question: If we compare erlotinib with the best adjuvant chemotherapy
regimens, is that the way to go?
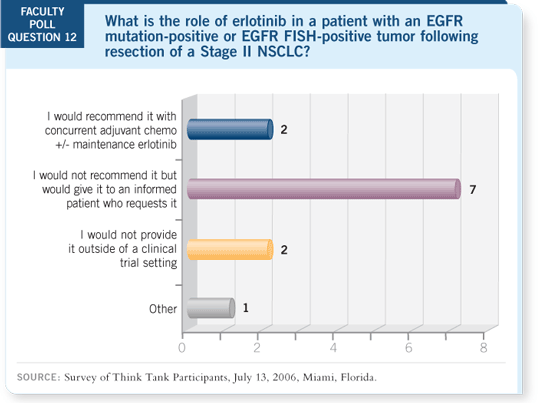
But I believe we’re talking about a selected population. In that situation, I
can’t go against the patient who has read all the data and wants to go that
route.
 DR EDELMAN: I want to see data. I have equipoise, which is why I can put a
patient on a Phase III trial. I don’t know the answer.
DR EDELMAN: I want to see data. I have equipoise, which is why I can put a
patient on a Phase III trial. I don’t know the answer.
 DR LYNCH: I’ve softened on this issue. I believe for patients who have
mutation-positive disease, you need to have a detailed discussion with them.
They’re not going to be able to wait for the Phase III trials to be conducted,
and obviously, I endorse the concept of Phase III trials.
DR LYNCH: I’ve softened on this issue. I believe for patients who have
mutation-positive disease, you need to have a detailed discussion with them.
They’re not going to be able to wait for the Phase III trials to be conducted,
and obviously, I endorse the concept of Phase III trials.
However, for that patient with mutation-positive disease, I have a long discussion
with them, and I don’t believe it’s crazy to consider adding erlotinib after
chemotherapy.
Select publications

