
 |
|||||||

| Tracks 1-12 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2, 4
![]() DR LOVE: Would you discuss the FLEX study you presented at ASCO?
DR LOVE: Would you discuss the FLEX study you presented at ASCO?
![]() PROF PIRKER: This trial aimed to demonstrate superior survival for chemotherapy
with cetuximab compared to chemotherapy alone in advanced
NSCLC (Pirker 2008; [1.1, 4.1]).
PROF PIRKER: This trial aimed to demonstrate superior survival for chemotherapy
with cetuximab compared to chemotherapy alone in advanced
NSCLC (Pirker 2008; [1.1, 4.1]).
The eligibility criteria included documented Stage IIIB disease with malignant pleural effusion or Stage IV disease. We attempted to evaluate EGFR expression by IHC in at least 100 cells from each patient. Patients with any positive EGFR expression in a tumor, as defined by positivity in one or more cells, were eligible. The assessment of EGFR expression was performed for 1,688 patients, of whom 85 percent fulfilled the criterion of at least one positive cell.
Patients with all histological subtypes were eligible. We included patients with ECOG PS 0 to 2. The other main inclusion criterion was that we did not screen for brain metastases. In the case of known brain metastases, patients were excluded. So it was a broad patient population.
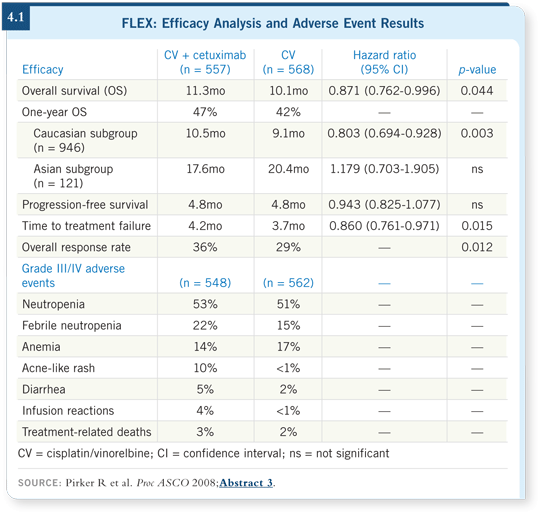
Cetuximab with chemotherapy demonstrated superior overall survival compared to chemotherapy alone, with a hazard ratio of 0.87 and a 30 percent risk reduction.
The median survival in the cetuximab arm was 11.3 months versus 10.1 months, and the one-year survival in the cetuximab/chemotherapy arm was 47 percent compared to 42 percent in the chemotherapy-alone arm for the overall population (4.1).
We did not see a significant difference in progression-free survival, however. We also analyzed time to treatment failure, and we observed a significant difference in favor of the cetuximab arm.
We conducted an exploratory subgroup analysis that indicated a benefit in all the subgroups analyzed except the Asian patients, which was a small population. The forest plot for the Asian subgroup was to the right, slightly above one, but with a broad confidence interval, so we can’t make any statement from it. For all the other groups, it was to the left.
Track 6
![]() DR LOVE: What do you think about the clinical implications of this
study?
DR LOVE: What do you think about the clinical implications of this
study?
![]() PROF PIRKER: I believe that the implications are that it will become one of
the standard treatments in the future, if not the standard treatment, at least
in Europe. I anticipate this because of the survival advantage reported with
cetuximab in such a broad population with all histological subtypes.
PROF PIRKER: I believe that the implications are that it will become one of
the standard treatments in the future, if not the standard treatment, at least
in Europe. I anticipate this because of the survival advantage reported with
cetuximab in such a broad population with all histological subtypes.
It’s also of importance to note that a cisplatin-based protocol was used, and I believe that cisplatin-based protocols are slightly superior to carboplatin-based protocols, particularly with regard to patient survival.
The addition of cetuximab to optimal chemotherapy further improves survival. I would consider cetuximab with cisplatin-based therapy as a new standard.
![]() DR LOVE: What about patients who would have met the eligibility criteria for
the ECOG-E4599 trial of paclitaxel/carboplatin with or without bevacizumab
(Sandler 2006)?
DR LOVE: What about patients who would have met the eligibility criteria for
the ECOG-E4599 trial of paclitaxel/carboplatin with or without bevacizumab
(Sandler 2006)?
![]() PROF PIRKER: Compared to the inclusion criteria for the ECOG trial of
chemotherapy with bevacizumab, we reached the same median survival in this
population of patients with adenocarcinomas.
PROF PIRKER: Compared to the inclusion criteria for the ECOG trial of
chemotherapy with bevacizumab, we reached the same median survival in this
population of patients with adenocarcinomas.
We reported an improvement of nearly two months, from 10.3 months median in the control arm to 12 months in the chemotherapy/cetuximab arm.
In this patient population, I would use cisplatin-based chemotherapy with cetuximab, based on the data with cetuximab improving survival with a cisplatin-based protocol and based on the fact that bevacizumab did not significantly improve overall survival in the AVAiL trial (Manegold 2007).
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Thomas J Lynch, MD
- Select publications
F Anthony Greco, MD
- Select publications
Robert Pirker, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity