
 |
|||||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 6-7
![]() DR LOVE: In clinical practice, how do you treat the patient with
metastatic NSCLC who is a nonsmoker?
DR LOVE: In clinical practice, how do you treat the patient with
metastatic NSCLC who is a nonsmoker?
![]() DR GRECO: I believe those patients should first receive oral tyrosine kinase
drugs — the EGFR inhibitors — and I prefer erlotinib. No cure exists for these
patients, and if they have an exon 19 or 21 mutation, which most of them have,
the median survival is close to two years with this agent (Riely 2006).
DR GRECO: I believe those patients should first receive oral tyrosine kinase
drugs — the EGFR inhibitors — and I prefer erlotinib. No cure exists for these
patients, and if they have an exon 19 or 21 mutation, which most of them have,
the median survival is close to two years with this agent (Riely 2006).
One could argue that we should administer chemotherapy first, but I don’t agree. Chemotherapy is more toxic, in general, and I’m not sure we gain anything by using it first. We need studies to determine whether we should use sequential therapies or combinations, but right now I would use erlotinib for these patients as a single drug for primary therapy.
![]() DR LOVE: How would you treat that same population in the adjuvant setting?
DR LOVE: How would you treat that same population in the adjuvant setting?
![]() DR GRECO: We have no data in the adjuvant setting, but I believe tumor
cells don’t care whether they’re in an overt metastatic or an adjuvant setting.
Micrometastatic disease is highly likely to respond to an agent like erlotinib if
the mutation is present, so I would somehow incorporate erlotinib.
DR GRECO: We have no data in the adjuvant setting, but I believe tumor
cells don’t care whether they’re in an overt metastatic or an adjuvant setting.
Micrometastatic disease is highly likely to respond to an agent like erlotinib if
the mutation is present, so I would somehow incorporate erlotinib.
However, in the adjuvant setting I would not use erlotinib as a single drug because we have data suggesting that chemotherapy improves survival in this setting, for instance, in Stage IIB NSCLC. Therefore, for such patients I would use chemotherapy and then add erlotinib, probably for a year.
![]() DR LOVE: Are you concerned about using a drug in practice that is being
studied in adjuvant clinical trials but has not yet been approved in that setting?
DR LOVE: Are you concerned about using a drug in practice that is being
studied in adjuvant clinical trials but has not yet been approved in that setting?
![]() DR GRECO: When you have a patient in front of you asking for advice, to be
disciplined to the point of saying he or she must either go on study or may not
receive the drug to me is like “copping out.” That would be essentially not
giving the patient an opinion about what to do.
DR GRECO: When you have a patient in front of you asking for advice, to be
disciplined to the point of saying he or she must either go on study or may not
receive the drug to me is like “copping out.” That would be essentially not
giving the patient an opinion about what to do.
One might hold the opinion that patients should not receive adjuvant erlotinib off study, but considering the data with this drug in the metastatic setting, I believe it would be inconsistent not to administer it in the adjuvant setting for micrometastatic disease. If it’s proven to be harmful, then I won’t use it in that setting anymore. However, I won’t second-guess myself now because I don’t have evidence that it will be harmful and, indeed, it might help.
Track 12
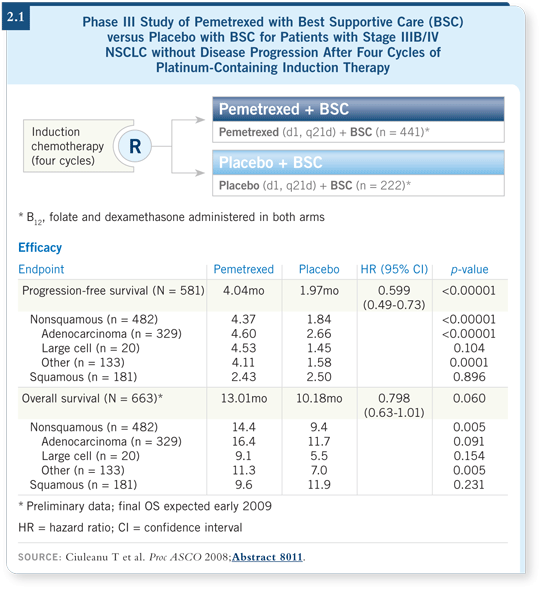
![]() DR LOVE: Can you comment on the data with maintenance pemetrexed
that were presented by Ciuleanu at ASCO?
DR LOVE: Can you comment on the data with maintenance pemetrexed
that were presented by Ciuleanu at ASCO?
![]() DR GRECO: This trial evaluated pemetrexed in patients whose disease had not
progressed after platinum-based induction chemotherapy. The patients were
randomly assigned to receive pemetrexed or no maintenance therapy. The trial
demonstrated an advantage with pemetrexed maintenance for the patients with
nonsquamous cell cancer (2.1).
DR GRECO: This trial evaluated pemetrexed in patients whose disease had not
progressed after platinum-based induction chemotherapy. The patients were
randomly assigned to receive pemetrexed or no maintenance therapy. The trial
demonstrated an advantage with pemetrexed maintenance for the patients with
nonsquamous cell cancer (2.1).
I found the data interesting and important considering the advanced disease trial that was presented at the International Association for the Study of Lung Cancer in Korea in September 2007 (Scagliotti 2007). That study showed that in advanced disease, first-line pemetrexed/cisplatin was superior to gemcitabine/cisplatin for patients with nonsquamous histologies.
I believe pemetrexed is likely to play a major role in the treatment of nonsquamous cell cancer, as first-line therapy and perhaps as maintenance therapy as well. It might be that because we’re using a better drug, we could use it in either setting and still obtain the same overall survival benefit. That’s my bias.
![]() DR LOVE: What chemotherapy regimen do you currently use for front-line
therapy in your practice?
DR LOVE: What chemotherapy regimen do you currently use for front-line
therapy in your practice?
![]() DR GRECO: I favor either gemcitabine/carboplatin or pemetrexed/carboplatin.
While I do use taxanes with carboplatin, I’m using them less often now off
study because of the toxicity issues.
DR GRECO: I favor either gemcitabine/carboplatin or pemetrexed/carboplatin.
While I do use taxanes with carboplatin, I’m using them less often now off
study because of the toxicity issues.
Gemcitabine/carboplatin is one of my preferred chemotherapy combinations because it’s as effective as other regimens and it’s well tolerated. Based on the emerging data, I also like pemetrexed/carboplatin for patients with nonsquamous histologies. I believe that combination is even better tolerated than gemcitabine/carboplatin and the results are equally good, if not better, in that population.
Maintenance pemetrexed will probably increase the survival of patients with nonsquamous histologies, so I would also consider that therapy for selected patients. However, to me, what we use in the front line is probably more important, and this is evolving. Pemetrexed will likely be one of the major drugs to use with a platinum in front-line therapy for nonsquamous cancer, and it’s likely to be soon.
Track 13
![]() DR LOVE: How do paclitaxel, docetaxel and nab paclitaxel compare in
terms of treating NSCLC?
DR LOVE: How do paclitaxel, docetaxel and nab paclitaxel compare in
terms of treating NSCLC?
![]() DR GRECO: I believe the verdict is still out on nab paclitaxel, but it may end
up being a better taxane than paclitaxel. I’m awaiting the data from the Phase
III CA031 trial comparing carboplatin/nab paclitaxel to carboplatin/paclitaxel
for patients with advanced NSCLC. Nab paclitaxel is easier to use and can be
administered over a shorter period of time.
DR GRECO: I believe the verdict is still out on nab paclitaxel, but it may end
up being a better taxane than paclitaxel. I’m awaiting the data from the Phase
III CA031 trial comparing carboplatin/nab paclitaxel to carboplatin/paclitaxel
for patients with advanced NSCLC. Nab paclitaxel is easier to use and can be
administered over a shorter period of time.
In breast cancer, nab paclitaxel clearly appears to be superior to paclitaxel, at least in the second-line setting, and maybe to docetaxel. While the verdict’s not been reached on nab paclitaxel, I expect that before long we will see a major dip in the use of the other taxanes in lung cancer. This is my opinion, and it’s partly because of the data that are emerging with the pemetrexed combinations.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Thomas J Lynch, MD
- Select publications
F Anthony Greco, MD
- Select publications
Robert Pirker, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity