
 |
|||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
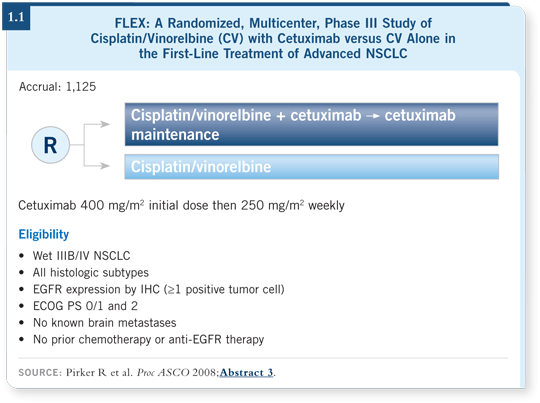
![]() DR LOVE: Can you discuss the FLEX trial that was presented by Robert
Pirker at ASCO?
DR LOVE: Can you discuss the FLEX trial that was presented by Robert
Pirker at ASCO?
![]() DR LYNCH: The FLEX study was a Phase III randomized trial for patients
with advanced NSCLC, who were randomly assigned to treatment with
cisplatin/vinorelbine alone or cisplatin/vinorelbine with weekly cetuximab
(Pirker 2008; [1.1]). The key selection criteria in FLEX were different from
those in a number of other trials. Patients were selected on the basis of positive
EGFR staining by immunohistochemistry (IHC). At least one cell had to be
EGFR-positive, which is a fairly liberal criterion. This excluded the approximately
15 percent of patients who did not have detectable EGFR expression.
DR LYNCH: The FLEX study was a Phase III randomized trial for patients
with advanced NSCLC, who were randomly assigned to treatment with
cisplatin/vinorelbine alone or cisplatin/vinorelbine with weekly cetuximab
(Pirker 2008; [1.1]). The key selection criteria in FLEX were different from
those in a number of other trials. Patients were selected on the basis of positive
EGFR staining by immunohistochemistry (IHC). At least one cell had to be
EGFR-positive, which is a fairly liberal criterion. This excluded the approximately
15 percent of patients who did not have detectable EGFR expression.
A statistically significant difference in response rate favored cetuximab. Most importantly, survival was prolonged, with median survival increasing from approximately 10.1 months to approximately 11.3 months for patients treated with chemotherapy and cetuximab.
Interestingly, no difference was apparent in progression-free survival between the two arms (4.1). That was a big surprise because an expectation exists for progression-free survival to trend in the same direction as overall survival. Still, I believe this was an important but somewhat modest benefit in the first study of cetuximab in this setting.
In my podium discussion of Professor Pirker’s presentation at ASCO, I raised the question of whether we should be using IHC staining. In the future, we will probably move away from that. However, right now, after a study with such a close margin of benefit, following the entrance criteria is important. I believe I’ll be using IHC in my practice to exclude patients who may not benefit from cetuximab.
Tracks 3-4
![]() DR LOVE: What are the practical clinical implications of the FLEX trial?
DR LOVE: What are the practical clinical implications of the FLEX trial?
![]() DR LYNCH: I believe cetuximab will be used for NSCLC. Patients and
doctors want options that improve outcome. Cetuximab with chemotherapy
improves outcome, similar to the way that bevacizumab with chemotherapy
improves outcome. I believe we’ll see bevacizumab used for bevacizumab-eligible
patients and cetuximab used for patients for whom bevacizumab is not
an option. Learning more about which biomarkers identify those who might
benefit from therapy may broaden the group of patients who are treated
with cetuximab.
DR LYNCH: I believe cetuximab will be used for NSCLC. Patients and
doctors want options that improve outcome. Cetuximab with chemotherapy
improves outcome, similar to the way that bevacizumab with chemotherapy
improves outcome. I believe we’ll see bevacizumab used for bevacizumab-eligible
patients and cetuximab used for patients for whom bevacizumab is not
an option. Learning more about which biomarkers identify those who might
benefit from therapy may broaden the group of patients who are treated
with cetuximab.
![]() DR LOVE: What are your thoughts on the risks and benefits of using cetuximab
for bevacizumab-eligible patients?
DR LOVE: What are your thoughts on the risks and benefits of using cetuximab
for bevacizumab-eligible patients?
![]() DR LYNCH: I still believe bevacizumab is a good drug. American oncologists
have shown a remarkable ability to use it safely. The toxicity reports on the
ARIES study, a registry trial of thousands of patients who have been treated
with chemotherapy and bevacizumab, are consistently better than those from
either ECOG-E4599 or AVAiL (Lynch 2008; Sandler 2006; Manegold 2007).
That suggests practicing physicians are selecting the right patients to treat with
bevacizumab. Bevacizumab is safe and active in patients with lung cancer, so I
don’t think that we should give up on bevacizumab at this point.
DR LYNCH: I still believe bevacizumab is a good drug. American oncologists
have shown a remarkable ability to use it safely. The toxicity reports on the
ARIES study, a registry trial of thousands of patients who have been treated
with chemotherapy and bevacizumab, are consistently better than those from
either ECOG-E4599 or AVAiL (Lynch 2008; Sandler 2006; Manegold 2007).
That suggests practicing physicians are selecting the right patients to treat with
bevacizumab. Bevacizumab is safe and active in patients with lung cancer, so I
don’t think that we should give up on bevacizumab at this point.
In the subset of Caucasian patients with adenocarcinomas who were treated with cetuximab in the FLEX trial, the benefit appears to be similar to that in ECOG-E4599 (Sandler 2006). However, that’s not a head-to-head comparison, so I believe one must be cautious. The real question is how we use them in combination, and that’s what the SWOG-S0819 trial will evaluate.
SWOG-S0819 is currently before the NCI, and according to the current design, bevacizumab-eligible patients will be randomly assigned to carboplatin/paclitaxel and bevacizumab or carboplatin/paclitaxel and bevacizumab/cetuximab. Bevacizumab-ineligible patients will be randomly assigned to carboplatin/paclitaxel or carboplatin/paclitaxel and cetuximab. This is a confirmatory study for carboplatin/paclitaxel, but in the bevacizumab-eligible population, we’ll learn whether two antibodies are better than one.
Track 12
![]() DR LOVE: What are your thoughts on vandetanib? How does it work and
where do you think it’s heading clinically?
DR LOVE: What are your thoughts on vandetanib? How does it work and
where do you think it’s heading clinically?
![]() DR LYNCH: Vandetanib is another drug that’s generating interest. It is a dual
kinase inhibitor, inhibiting both EGF and VEGF, though I believe most of
its activity comes from its VEGF inhibition. Vandetanib is being evaluated in
Phase III trials in two settings: First, as monotherapy compared to erlotinib in
a large trial led by Ron Natale (Study 57) and second, in a trial led by John Heymach of docetaxel versus docetaxel/vandetanib in the second-line setting
(ZODIAC).
DR LYNCH: Vandetanib is another drug that’s generating interest. It is a dual
kinase inhibitor, inhibiting both EGF and VEGF, though I believe most of
its activity comes from its VEGF inhibition. Vandetanib is being evaluated in
Phase III trials in two settings: First, as monotherapy compared to erlotinib in
a large trial led by Ron Natale (Study 57) and second, in a trial led by John Heymach of docetaxel versus docetaxel/vandetanib in the second-line setting
(ZODIAC).
Personally, I have confidence that the docetaxel/vandetanib trial will be positive. It’s a lot to ask of vandetanib to be better than erlotinib. However, the docetaxel/vandetanib trial is exciting, and if it’s positive, this could end up setting a new standard for second-line lung cancer.
Tracks 16-18
![]() DR LOVE: Let’s talk about adjuvant therapy. First, how are you
approaching the decision of what chemotherapy to utilize?
DR LOVE: Let’s talk about adjuvant therapy. First, how are you
approaching the decision of what chemotherapy to utilize?
![]() DR LYNCH: As my default, I prefer cisplatin/docetaxel because it can be
administered relatively easily, one day every three weeks, and it’s well tolerated.
We do use carboplatin-based regimens with some patients. In a real-world
situation, such as when you’re seeing a patient who is 76 years old, has
a creatinine level of 1.7 milligrams per deciliter and perhaps had a myocardial
infarction and a stroke, you will not necessarily administer cisplatin. I believe
more carboplatin is being used than people may recognize.
DR LYNCH: As my default, I prefer cisplatin/docetaxel because it can be
administered relatively easily, one day every three weeks, and it’s well tolerated.
We do use carboplatin-based regimens with some patients. In a real-world
situation, such as when you’re seeing a patient who is 76 years old, has
a creatinine level of 1.7 milligrams per deciliter and perhaps had a myocardial
infarction and a stroke, you will not necessarily administer cisplatin. I believe
more carboplatin is being used than people may recognize.
![]() DR LOVE: What about patients with EGFR mutations?
DR LOVE: What about patients with EGFR mutations?
![]() DR LYNCH: We are accruing to a study evaluating the role of adjuvant
erlotinib for patients with EGFR mutations (NCT00567359). Fortunately,
the trial’s inclusion criteria are fairly liberal, and I’m encouraging all of our
eligible patients to enroll. The trial involves two years of adjuvant erlotinib.
DR LYNCH: We are accruing to a study evaluating the role of adjuvant
erlotinib for patients with EGFR mutations (NCT00567359). Fortunately,
the trial’s inclusion criteria are fairly liberal, and I’m encouraging all of our
eligible patients to enroll. The trial involves two years of adjuvant erlotinib.
Outside the setting of a study, I believe it is permissible to consider a treatment like this. We know the response rates in this population are extraordinarily high. The issue is, we don’t know what the long-term side effects are or the optimal duration of therapy. Patients develop significant rash. Many people experience loose bowels. For patients who are really benefiting, the rash will burn out. It will not stay at that same level of intensity that you find in the first two months. In advanced disease, I have patients who have been on gefitinib and erlotinib for four, five, six, seven years.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Thomas J Lynch, MD
- Select publications
F Anthony Greco, MD
- Select publications
Robert Pirker, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity