
 |
|||||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Can you discuss the recently published practice guidelines on
adjuvant therapy developed by Cancer Care Ontario and the American
Society of Clinical Oncology (Pisters 2007)?
DR LOVE: Can you discuss the recently published practice guidelines on
adjuvant therapy developed by Cancer Care Ontario and the American
Society of Clinical Oncology (Pisters 2007)?
![]() DR KRIS: First I would like to point out the unanimity of the group in
agreeing that adjuvant therapy — particularly adjuvant cisplatin-based chemotherapy
— improves survival. It is important to deliver that message.
DR KRIS: First I would like to point out the unanimity of the group in
agreeing that adjuvant therapy — particularly adjuvant cisplatin-based chemotherapy
— improves survival. It is important to deliver that message.
The devil is in the details. Agreement was reached that the data are strong for Stage II and Stage IIIA disease, and the guidelines represent a standard. However, in some areas the recommendations are not as strong. One of these areas is Stage IB disease — only one clinical trial specifically addressed that group (CALGB-9633), and it did not show a survival benefit (Strauss 2006).
In other adjuvant trials that did show a benefit — IALT, CAN-NCIC-BR10 and the ANITA trial — the primary endpoint was improvement in five-year survival for the entire study population. All those trials included patients with Stage IB disease, and they were all convincingly positive (Arriagada 2004; Winton 2005; Douillard 2006).
![]() DR LOVE: How do you treat patients with Stage IB disease in your practice?
DR LOVE: How do you treat patients with Stage IB disease in your practice?
![]() DR KRIS: I believe these patients should be offered adjuvant therapy, and I
would probably offer it to patients with Stage IA disease also. Even with the
new staging system, the five-year survival for these patients is such that we’d
recommend adjuvant therapy if it were breast cancer.
DR KRIS: I believe these patients should be offered adjuvant therapy, and I
would probably offer it to patients with Stage IA disease also. Even with the
new staging system, the five-year survival for these patients is such that we’d
recommend adjuvant therapy if it were breast cancer.
Track 4
![]() DR LOVE: What are your thoughts about ECOG-E1505, an ongoing trial
evaluating three different types of cisplatin-based chemotherapy with or
without bevacizumab?
DR LOVE: What are your thoughts about ECOG-E1505, an ongoing trial
evaluating three different types of cisplatin-based chemotherapy with or
without bevacizumab?
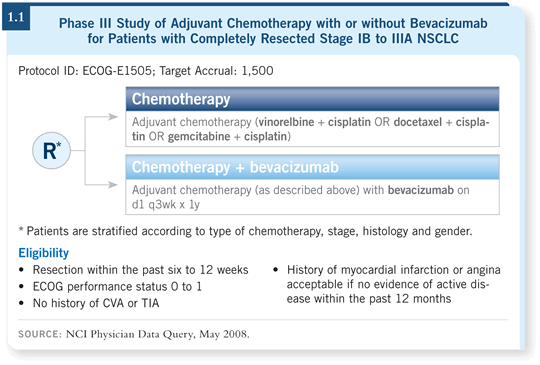
![]() DR KRIS: This trial evaluates cisplatin in combination with vinorelbine,
gemcitabine or docetaxel, with or without bevacizumab (1.1).
DR KRIS: This trial evaluates cisplatin in combination with vinorelbine,
gemcitabine or docetaxel, with or without bevacizumab (1.1).
We need to consider that this trial has a couple of caveats. One is our ability to administer each of those regimens. I expected that docetaxel/cisplatin might be superior because in the TAX-326 trial, that regimen showed improved survival and response over vinorelbine/cisplatin in the metastatic setting (Fossella 2003). However, we conducted two trials with the docetaxel/cisplatin regimen used in the ECOG trial, and while we thought it was a great idea, we were not able to deliver it.
The other caveat is the likelihood for greater myelosuppression when combining bevacizumab with chemotherapy, as seen in the Sandler trial, so we need to watch out for that (Sandler 2006).
Track 14
![]() DR LOVE: What are your thoughts about nanoparticle albumin-bound
(nab) paclitaxel in non-small cell lung cancer?
DR LOVE: What are your thoughts about nanoparticle albumin-bound
(nab) paclitaxel in non-small cell lung cancer?
![]() DR KRIS: The use of nab paclitaxel in breast cancer is fairly extensive, suggesting that it is at least equivalent and probably better than paclitaxel. Additionally,
it has one clear toxicity advantage, which is the lack of hypersensitivity
reactions that are frightening to patients and, on rare occasions, can be lethal.
DR KRIS: The use of nab paclitaxel in breast cancer is fairly extensive, suggesting that it is at least equivalent and probably better than paclitaxel. Additionally,
it has one clear toxicity advantage, which is the lack of hypersensitivity
reactions that are frightening to patients and, on rare occasions, can be lethal.
The other toxicities — alopecia, neutropenia and neurotoxicity — are comparable. To me, if good evidence of equivalence were available, with the safety advantage, nab paclitaxel would have an edge over the other taxanes.
Track 16
![]() DR LOVE: What are your thoughts about the combination of an EGFR
tyrosine kinase inhibitor like erlotinib with bevacizumab?
DR LOVE: What are your thoughts about the combination of an EGFR
tyrosine kinase inhibitor like erlotinib with bevacizumab?
![]() DR KRIS: Much empirical evidence supports that approach — they are two
active agents with completely different side-effect profiles and mechanisms of
action. Clearly it can be done, and many combine them routinely.
DR KRIS: Much empirical evidence supports that approach — they are two
active agents with completely different side-effect profiles and mechanisms of
action. Clearly it can be done, and many combine them routinely.
![]() DR LOVE: In what clinical scenarios are they combined?
DR LOVE: In what clinical scenarios are they combined?
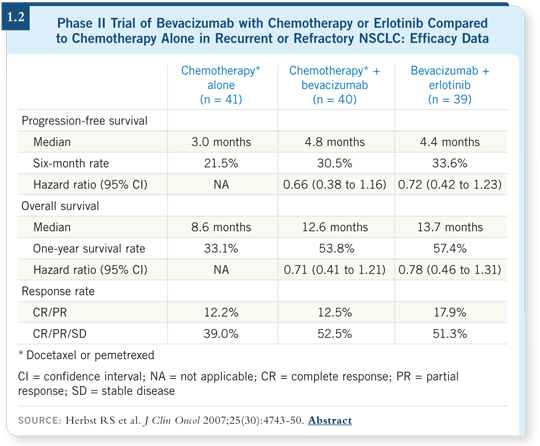
![]() DR KRIS: Physicians use it as second-line therapy, although that was more
common before bevacizumab was widely available. The safety of combining
these agents is clear in the studies that have been reported (Herbst 2007; [1.2]).
We also use the combination up front for some patients who are candidates for
both agents.
DR KRIS: Physicians use it as second-line therapy, although that was more
common before bevacizumab was widely available. The safety of combining
these agents is clear in the studies that have been reported (Herbst 2007; [1.2]).
We also use the combination up front for some patients who are candidates for
both agents.
It makes sense, particularly for a patient who has an EGFR mutation or a high likelihood of having an EGFR mutation, such as a woman who’s a never smoker. That patient has at least a 50-50 chance of having a mutation, and it makes sense to administer chemotherapy with bevacizumab and erlotinib.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Mark G Kris, MD
- Select publications
Roman Perez-Soler, MD
- Select publications
Julie R Brahmer, MD
- Select publications
Philip Bonomi, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity