
 |
|||||||

| Tracks 1-8 | ||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you describe the recent results of the trial evaluating cisplatin
with either pemetrexed or gemcitabine in advanced disease?
DR LOVE: Can you describe the recent results of the trial evaluating cisplatin
with either pemetrexed or gemcitabine in advanced disease?
![]() DR BONOMI: As we move forward, chemotherapy will be used in different,
more targeted fashions as was seen in the Phase III randomized study of
pemetrexed/cisplatin versus gemcitabine/cisplatin.
DR BONOMI: As we move forward, chemotherapy will be used in different,
more targeted fashions as was seen in the Phase III randomized study of
pemetrexed/cisplatin versus gemcitabine/cisplatin.
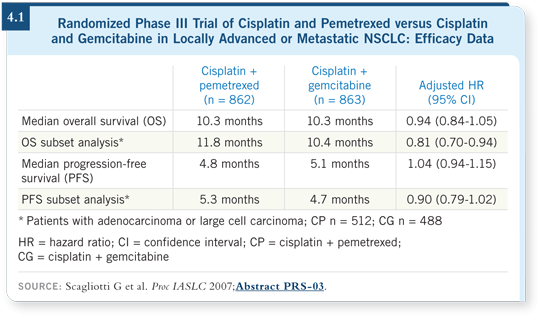
This was a 1,700-patient study, of which approximately 1,200 patients had a nonsquamous cell diagnosis. A subset analysis of patients with adenocarcinomas and large cell histology reported significantly longer overall survival with pemetrexed versus gemcitabine (Scagliotti 2007; [4.1]).
It has always been said that histology doesn’t make any difference to the effectiveness of chemotherapy in relation to response or survival. These results flew in the face of convention and made everybody say, “Wait a minute.” Many people remain skeptical, but increasing data will suggest that pemetrexed works better in adenocarcinoma.
The hazard ratio was approximately 0.8, which wasn’t a home run. However, without doing anything differently except selecting the patients, there seems to be an advantage with pemetrexed (Hanauske 2007).
Treatment of Stage IV disease has always been about trying to relieve symptoms and prolong life. It is a testing ground for new ideas that we hope to move into earlier-stage disease — locally advanced disease and ultimately into the adjuvant setting — which we hope will translate into longer survival.
Track 2
![]() DR LOVE: About what fraction of patients with extensive NSCLC meet
the entry criteria for the trials evaluating bevacizumab?
DR LOVE: About what fraction of patients with extensive NSCLC meet
the entry criteria for the trials evaluating bevacizumab?
![]() DR BONOMI: ECOG-E4599 reported longer progression-free survival with
chemotherapy and bevacizumab versus chemotherapy alone (Sandler 2006).
The most important consideration, however, is the current exclusion criteria.
We don’t use bevacizumab to treat patients with brain metastases, squamous
cell carcinoma, hemoptysis or those on anticoagulation.
DR BONOMI: ECOG-E4599 reported longer progression-free survival with
chemotherapy and bevacizumab versus chemotherapy alone (Sandler 2006).
The most important consideration, however, is the current exclusion criteria.
We don’t use bevacizumab to treat patients with brain metastases, squamous
cell carcinoma, hemoptysis or those on anticoagulation.
My impression, though, is that only 35 or 40 percent of the people who walk through your door with Stage IV disease meet these criteria. However, this might change because current studies are evaluating bevacizumab in patients with brain metastasis.
And although improvements were modest on ECOG-E4599, we are now moving into the adjuvant setting with the ongoing ECOG-E1505 Intergroup study of postoperative chemotherapy with or without bevacizumab (1.1).
On that study, we do not have as many exclusions, and the issue is whether or not bevacizumab will have a greater impact in the treatment of early-stage disease.
Track 3
![]() DR LOVE: What’s your treatment algorithm for patients with advanced
NSCLC who are nonsmokers or who have EGFR mutations?
DR LOVE: What’s your treatment algorithm for patients with advanced
NSCLC who are nonsmokers or who have EGFR mutations?
![]() DR BONOMI: We don’t know the answer yet, but I and many other people
would treat such patients with an EGFR TKI first, then proceed to chemotherapy
later. I wouldn’t administer them together, although some clinicians
would consider using the combination.
DR BONOMI: We don’t know the answer yet, but I and many other people
would treat such patients with an EGFR TKI first, then proceed to chemotherapy
later. I wouldn’t administer them together, although some clinicians
would consider using the combination.
I would treat with the EGFR TKI and evaluate the response. Not all patients respond, but they have a relatively high response rate — higher than with chemotherapy.
![]() DR LOVE: For the patient who responds, what would you do after disease
progression?
DR LOVE: For the patient who responds, what would you do after disease
progression?
![]() DR BONOMI: I would use chemotherapy with bevacizumab, if appropriate.
DR BONOMI: I would use chemotherapy with bevacizumab, if appropriate.
![]() DR LOVE: Have you used erlotinib with bevacizumab?
DR LOVE: Have you used erlotinib with bevacizumab?
![]() DR BONOMI: Yes. In fact, I had a patient who had a large mass in his lung,
multiple nodules, PS 1.5 and extensive bone metastases.
DR BONOMI: Yes. In fact, I had a patient who had a large mass in his lung,
multiple nodules, PS 1.5 and extensive bone metastases.
A friend advised him to opt for erlotinib and bevacizumab with no chemotherapy, and we did that. He had an unbelievable response and went into a remission for a year and a half.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Mark G Kris, MD
- Select publications
Roman Perez-Soler, MD
- Select publications
Julie R Brahmer, MD
- Select publications
Philip Bonomi, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity