
 |
|||||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you comment on predictors of response to EGFR TKIs?
DR LOVE: Can you comment on predictors of response to EGFR TKIs?
![]() DR BRAHMER: Some investigators believe that EGFR mutations are the major
predictors of response, while others believe that EGFR gene expression, as
measured by FISH, determines benefit from tyrosine kinase inhibitors. We
know that patients treated with EGFR inhibitors will respond better if they
have an EGFR mutation, but will they live longer? The large Canadian trial
CAN-NCIC-BR21, which evaluated erlotinib versus placebo, retrospectively
addressed this issue, and patients with EGFR mutations did not live any longer
than those without the mutation when treated with erlotinib (Shepherd 2007).
DR BRAHMER: Some investigators believe that EGFR mutations are the major
predictors of response, while others believe that EGFR gene expression, as
measured by FISH, determines benefit from tyrosine kinase inhibitors. We
know that patients treated with EGFR inhibitors will respond better if they
have an EGFR mutation, but will they live longer? The large Canadian trial
CAN-NCIC-BR21, which evaluated erlotinib versus placebo, retrospectively
addressed this issue, and patients with EGFR mutations did not live any longer
than those without the mutation when treated with erlotinib (Shepherd 2007).
However, patients treated with erlotinib who had increased EGFR gene expression as determined by FISH did live longer (Shepherd 2007). Data from the INTEREST study, evaluating gefitinib versus docetaxel, may reverse those findings (Douillard 2007).
The first-line trials evaluating erlotinib in patients with EGFR mutations will answer whether we should move erlotinib to the first-line setting for those patients. I don’t believe the mutations will indicate whether a patient will live longer with erlotinib versus another treatment, but those patients with EGFR mutations probably need erlotinib up front rather than chemotherapy.
Track 4
![]() DR LOVE: Can you discuss the data in NSCLC with vandetanib, which
targets both the EGFR and VEGF pathways?
DR LOVE: Can you discuss the data in NSCLC with vandetanib, which
targets both the EGFR and VEGF pathways?
![]() DR BRAHMER: Dr John Heymach from MD Anderson has led much of the
research on this agent and has presented interesting data combining chemotherapy
and vandetanib. Vandetanib is both an anti-angiogenic and an inhibitor
of EGFR, depending on the dose. Vandetanib at 100 milligrams per day
has both anti-EGFR and anti-VEGF activity.
DR BRAHMER: Dr John Heymach from MD Anderson has led much of the
research on this agent and has presented interesting data combining chemotherapy
and vandetanib. Vandetanib is both an anti-angiogenic and an inhibitor
of EGFR, depending on the dose. Vandetanib at 100 milligrams per day
has both anti-EGFR and anti-VEGF activity.
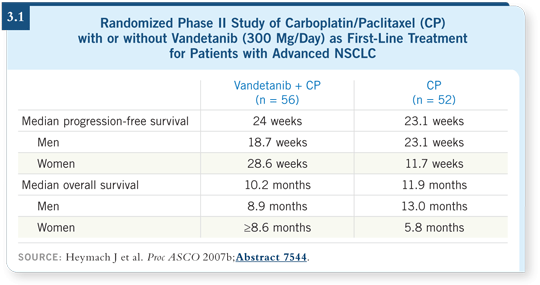
The higher dose of 300 milligrams per day — at least when combined with chemotherapy — did not improve progression-free survival (Heymach 2007b; [3.1]). These findings have led to a large Phase III trial, which will be evaluating vandetanib at 100 milligrams per day with chemotherapy versus chemotherapy alone as second-line therapy.
Tracks 6, 8
![]() DR LOVE: Can you provide an update on the ECOG-E4599 trial, which
evaluated bevacizumab in advanced NSCLC (Sandler 2006)?
DR LOVE: Can you provide an update on the ECOG-E4599 trial, which
evaluated bevacizumab in advanced NSCLC (Sandler 2006)?
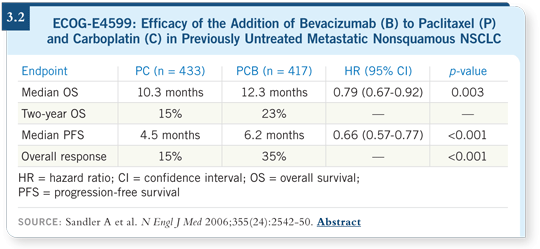
![]() DR BRAHMER: Patients with nonsquamous cell metastatic NSCLC were
randomly assigned to first-line therapy with carboplatin/paclitaxel with or without bevacizumab at 15 mg/kg. The patients treated with carboplatin/paclitaxel/bevacizumab experienced a significant improvement in overall survival
compared to those who received chemotherapy alone (Sandler 2006; [3.2]).
DR BRAHMER: Patients with nonsquamous cell metastatic NSCLC were
randomly assigned to first-line therapy with carboplatin/paclitaxel with or without bevacizumab at 15 mg/kg. The patients treated with carboplatin/paclitaxel/bevacizumab experienced a significant improvement in overall survival
compared to those who received chemotherapy alone (Sandler 2006; [3.2]).
However, in this study, elderly patients who were treated with chemotherapy and bevacizumab experienced increased toxicities with no improvement in survival compared to those treated with chemotherapy alone. For patients who are more prone to complications because of a drop in blood counts or any sign that they might be prone to bleeding, I would avoid using the three-drug regimen, not particularly because of age but certainly because of comorbidities, lower physical activity and the potential for bleeding.
![]() DR LOVE: If you see a patient in his or her seventies who otherwise meets the
criteria for E4599, do you have any hesitation about using bevacizumab?
DR LOVE: If you see a patient in his or her seventies who otherwise meets the
criteria for E4599, do you have any hesitation about using bevacizumab?
![]() DR BRAHMER: Absolutely not. I’d have more hesitation if they were in their
fifties and had a history of active coronary artery disease or even leg claudication.
I’d be more worried about those patients than the active 70-year-old
with no other health problems.
DR BRAHMER: Absolutely not. I’d have more hesitation if they were in their
fifties and had a history of active coronary artery disease or even leg claudication.
I’d be more worried about those patients than the active 70-year-old
with no other health problems.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Mark G Kris, MD
- Select publications
Roman Perez-Soler, MD
- Select publications
Julie R Brahmer, MD
- Select publications
Philip Bonomi, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity