
| Tracks 1-14 |
| Track 1 |
Introduction |
| Track 2 |
ECOG-E4599: Efficacy and toxicity of carboplatin/paclitaxel with or without bevacizumab for advanced nonsquamous-cell NSCLC |
| Track 3 |
Potential factors involved in bevacizumab-associated hemoptysis |
| Track 4 |
Use of bevacizumab in the metastatic setting |
| Track 5 |
Risk of bevacizumab-associated hemoptysis and use of bevacizumab in patients with treated brain metastases |
| Track 6 |
Gender differences in lung cancer and response to treatment |
| Track 7 |
Clinical trial strategies in small cell lung cancer (SCLC) |
| Track 8 |
ECOG-E1505 adjuvant trial of chemotherapy with or without bevacizumab in Stage IB-IIIA NSCLC |
|
| Track 9 |
Adjuvant chemotherapy in Stage IB NSCLC: Update of CALGB-9633 |
| Track 10 |
Use of standard-dose chemotherapy concurrently with radiation therapy in the treatment of Stage IIIB NSCLC |
| Track 11 |
Tolerability of consolidation
docetaxel after concurrent
chemoradiation therapy for Stage
IIIB NSCLC |
| Track 12 |
Factors influencing patient acceptance of adjuvant chemotherapy for NSCLC |
| Track 13 |
Studies evaluating radiation therapy/bevacizumab for Stage III disease |
| Track 14 |
Factors in decision-making about the use of the EGFR TKI erlotinib |
|
|
Select Excerpts from the Interview
Tracks 2-3
 DR LOVE:
DR LOVE: Would you discuss ECOG-E4599, which was the basis for the
new adjuvant trial evaluating chemotherapy and bevacizumab?
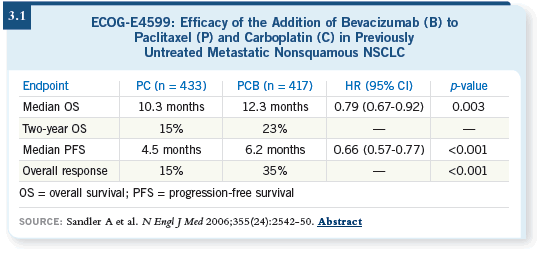
 DR SCHILLER: Based on the Phase II study that came out of Vanderbilt
(Johnson 2004), ECOG recently completed and published our randomized
Phase III study (E4599) in the first-line metastatic setting, in which patients
with nonsquamous non-small cell carcinoma of the lung who had undergone no prior chemotherapy were randomly assigned to receive either carboplatin/paclitaxel or carboplatin/paclitaxel and bevacizumab. A few more than 870
patients were assigned. We found a significant improvement in response rate,
progression-free survival and overall survival for the bevacizumab-containing
arm (Sandler 2006; [3.1]).
DR SCHILLER: Based on the Phase II study that came out of Vanderbilt
(Johnson 2004), ECOG recently completed and published our randomized
Phase III study (E4599) in the first-line metastatic setting, in which patients
with nonsquamous non-small cell carcinoma of the lung who had undergone no prior chemotherapy were randomly assigned to receive either carboplatin/paclitaxel or carboplatin/paclitaxel and bevacizumab. A few more than 870
patients were assigned. We found a significant improvement in response rate,
progression-free survival and overall survival for the bevacizumab-containing
arm (Sandler 2006; [3.1]).
 DR LOVE: Can you discuss the side effects and toxicity?
DR LOVE: Can you discuss the side effects and toxicity?
 DR SCHILLER: We were concerned about hemoptysis. In the randomized
Phase II study, the incidence of severe or fatal hemoptysis was greater than 30
percent among patients with squamous cell carcinoma (SCC). For that reason,
we excluded patients with SCC. The rate of clinically significant bleeding was
4.4 percent in the bevacizumab arm compared to 0.7 percent in the chemotherapy
arm. This is not what we would like it to be, but it is potentially
manageable in this group of patients with fatal disease.
DR SCHILLER: We were concerned about hemoptysis. In the randomized
Phase II study, the incidence of severe or fatal hemoptysis was greater than 30
percent among patients with squamous cell carcinoma (SCC). For that reason,
we excluded patients with SCC. The rate of clinically significant bleeding was
4.4 percent in the bevacizumab arm compared to 0.7 percent in the chemotherapy
arm. This is not what we would like it to be, but it is potentially
manageable in this group of patients with fatal disease.
The incidence of hypertension and proteinuria, which are class effects associated
with these agents, was also higher. It is interesting that neutropenia and
febrile neutropenia also occurred more in the bevacizumab arm. That’s not
something we were expecting. In preclinical models, VEGF has been shown
to be an immune stimulator, so it enhances the activity of immune cells. We’re
hypothesizing that bevacizumab inhibits that activity, thus increasing the
incidence of neutropenia.
Tracks 4-5
 DR LOVE:
DR LOVE: What is your clinical approach to the use of bevacizumab for
metastatic disease?
 DR SCHILLER: I use the ECOG-E4599 eligibility criteria: no brain metastases,
no SCC, no hemoptysis, no thromboembolic abnormalities and no anticoagulants.
Off study, for patients who meet the eligibility criteria, I routinely offer
bevacizumab as part of the treatment.
DR SCHILLER: I use the ECOG-E4599 eligibility criteria: no brain metastases,
no SCC, no hemoptysis, no thromboembolic abnormalities and no anticoagulants.
Off study, for patients who meet the eligibility criteria, I routinely offer
bevacizumab as part of the treatment.
Bevacizumab has been well tolerated, with the possible exception of hypertension, but that’s always been easily manageable. We’ve had no problems in that
regard, and we follow the typical hypertension management.
 DR LOVE: For what duration do you continue chemotherapy and bevacizumab?
DR LOVE: For what duration do you continue chemotherapy and bevacizumab?
 DR SCHILLER: I follow the E4599 guidelines: Stop the chemotherapy after
six cycles and continue the bevacizumab. A big question, which is currently
unanswered, is what to do when the disease progresses. If the patient is already
on bevacizumab, do you continue it? Clearly we need clinical studies to
answer that, particularly given the expense of this drug.
DR SCHILLER: I follow the E4599 guidelines: Stop the chemotherapy after
six cycles and continue the bevacizumab. A big question, which is currently
unanswered, is what to do when the disease progresses. If the patient is already
on bevacizumab, do you continue it? Clearly we need clinical studies to
answer that, particularly given the expense of this drug.
 DR LOVE: What about the use of other chemotherapeutic agents in combination
with bevacizumab?
DR LOVE: What about the use of other chemotherapeutic agents in combination
with bevacizumab?
 DR SCHILLER: Bevacizumab appears to work in colorectal carcinoma and in
breast cancer. I don’t believe it’s specific to any one type of chemotherapy, so I
have no problem using it with other drugs besides carboplatin and paclitaxel.
DR SCHILLER: Bevacizumab appears to work in colorectal carcinoma and in
breast cancer. I don’t believe it’s specific to any one type of chemotherapy, so I
have no problem using it with other drugs besides carboplatin and paclitaxel.
Track 8
 DR LOVE:
DR LOVE: Can you discuss the upcoming adjuvant trial that will be
conducted by ECOG?
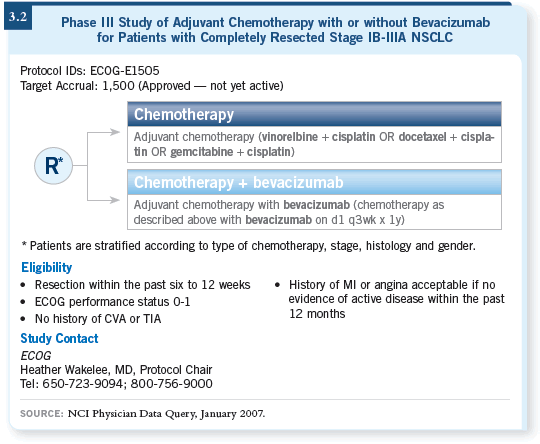
 DR SCHILLER: ECOG-E1505 will be a Phase III trial for patients with
selected Stage IB to IIIA NSCLC, who will be randomly assigned to four
cycles of chemotherapy versus four cycles of chemotherapy and up to one year
of bevacizumab (3.2).
DR SCHILLER: ECOG-E1505 will be a Phase III trial for patients with
selected Stage IB to IIIA NSCLC, who will be randomly assigned to four
cycles of chemotherapy versus four cycles of chemotherapy and up to one year
of bevacizumab (3.2).
Eligible patients will have Stage IB to IIIA disease, with IB tumors greater
than four centimeters in size. The reason for that is based on a subset analysis
CALGB conducted of their adjuvant study, in which patients with larger Stage
IB tumors were the ones who seemed to benefit (Strauss 2006). We’ll apply
the typical bevacizumab exclusion criteria. Patients will be allowed to have
had SCC, however, because the disease will be removed. It is hoped that the
histology will not be important if it’s not there.
 DR LOVE: What kind of chemotherapy will be allowed?
DR LOVE: What kind of chemotherapy will be allowed?
 DR SCHILLER: To some degree, it’s “dealer’s choice.” The referring physician
can choose among cisplatin/gemcitabine, cisplatin/docetaxel and cisplatin/vinorelbine.
DR SCHILLER: To some degree, it’s “dealer’s choice.” The referring physician
can choose among cisplatin/gemcitabine, cisplatin/docetaxel and cisplatin/vinorelbine.
Track 14
 DR LOVE:
DR LOVE: Let’s talk about another targeted therapy, erlotinib. To what
extent do you consider factors such as nonsmoking and EGFR mutation
status in deciding when to use erlotinib?
 DR SCHILLER: We have not been obtaining EGFR mutation status as a
standard rule because of all the other data that seem to suggest that EGFR
overexpression, determined either by protein or by FISH analysis, is also a strong predictor of benefit (Tsao 2005; Paez 2004). It may not predict
dramatic response rates, but it seems to predict stable disease and improved
survival. So we’ve been using erlotinib in our second-line therapy.
DR SCHILLER: We have not been obtaining EGFR mutation status as a
standard rule because of all the other data that seem to suggest that EGFR
overexpression, determined either by protein or by FISH analysis, is also a strong predictor of benefit (Tsao 2005; Paez 2004). It may not predict
dramatic response rates, but it seems to predict stable disease and improved
survival. So we’ve been using erlotinib in our second-line therapy.
We’ve been utilizing clinical factors along with how well patients responded
to first-line therapy. If someone’s disease progresses through first-line therapy,
we are more likely to use erlotinib in the second line rather than go on to a
second-line cytotoxic. Or if the patient is a woman, a nonsmoker, is of Asian
descent or has bronchoalveolar carcinoma, that too would push us to use
erlotinib as a second- or third-line therapy.
Select Publications

