
| Tracks 1-14 |
| Track 1 |
Introduction |
| Track 2 |
Adjustment for competing causes of mortality in lung cancer and feasibility of the development of an Adjuvant! Online-type model in lung cancer |
| Track 3 |
Impact of CALGB-9633 adjuvant trial on clinical practice |
| Track 4 |
Role of surgical expertise and procedures in the prognosis of resected NSCLC |
| Track 5 |
Importance of thoracic oncology surgeons in the treatment of NSCLC |
| Track 6 |
Use of cisplatin/docetaxel versus cisplatin/vinorelbine as adjuvant therapy |
| Track 7 |
Bevacizumab-associated toxicity in ECOG-E4599 |
| Track 8 |
Potential side effects and toxicity associated with adjuvant bevacizumab |
|
| Track 9 |
Challenges in the completion of adjuvant clinical trials in lung cancer |
| Track 10 |
Erlotinib for select patients with NSCLC in the adjuvant setting |
| Track 11 |
Differences in decision-making with regard to adjuvant treatment of breast and lung cancer |
| Track 12 |
Cavitation and bleeding and bevacizumab-associated antitumor effect |
| Track 13 |
Case discussion: An 80-year-old man with Stage IIIB NSCLC and preexisting peripheral neuropathy |
| Track 14 |
Use of the SWOG-S9504 regimen of chemoradiation therapy followed by consolidation docetaxel for Stage IIIB disease |
|
|
Select Excerpts from the Discussion
Track 2
 DR LOVE:
DR LOVE: Peter, can you comment on the competing causes of mortality
in patients with lung cancer?
 DR RAVDIN: The default estimates of competing mortality by age are higher
in patients with lung cancer than with other solid tumors. In the SEER data,
patients with lung cancer did slightly worse than an age-matched group for
competing mortality, which isn’t surprising. You see increased deaths from
cardiovascular disease and COPD-like illnesses in these patients.
DR RAVDIN: The default estimates of competing mortality by age are higher
in patients with lung cancer than with other solid tumors. In the SEER data,
patients with lung cancer did slightly worse than an age-matched group for
competing mortality, which isn’t surprising. You see increased deaths from
cardiovascular disease and COPD-like illnesses in these patients.
 DR LOVE: Vince, in breast cancer we use the concept of relative risk reduction
in the Adjuvant! Online model. Do we have enough data in lung cancer to do
the same?
DR LOVE: Vince, in breast cancer we use the concept of relative risk reduction
in the Adjuvant! Online model. Do we have enough data in lung cancer to do
the same?
 DR MILLER: We have a body of data with which we can start to do it, but
the quality of the data is not great. I think we need to enhance the data. We
always lag behind breast cancer by a few years, and this may be no exception.
DR MILLER: We have a body of data with which we can start to do it, but
the quality of the data is not great. I think we need to enhance the data. We
always lag behind breast cancer by a few years, and this may be no exception.
 DR LOVE: Corey, according to our Patterns of Care surveys, more than half
of the oncologists in the United States are using Adjuvant! Online for breast
cancer. The use in colon cancer is about one quarter of what it is in breast
cancer. Do you think Adjuvant! Online is going to have a future in lung
cancer in the next couple of years?
DR LOVE: Corey, according to our Patterns of Care surveys, more than half
of the oncologists in the United States are using Adjuvant! Online for breast
cancer. The use in colon cancer is about one quarter of what it is in breast
cancer. Do you think Adjuvant! Online is going to have a future in lung
cancer in the next couple of years?
 DR LANGER: I expect it will, but we need more trials. It’s not so much that
the quality of the data is not good — it’s the quantity of data that’s lacking. In
the modern era, we only have five or six trials.
DR LANGER: I expect it will, but we need more trials. It’s not so much that
the quality of the data is not good — it’s the quantity of data that’s lacking. In
the modern era, we only have five or six trials.
Many nuances factor into our therapeutic decision-making, particularly in
lung cancer — the patient’s comorbidities and performance status. This is a
population that’s generally older and a bit more ill than patients with breast
cancer. So the data from a cooperative group or a European trial do not necessarily
extrapolate to our patients.
 DR RAVDIN: The average patients with lung cancer behave as if they’re three
to five years older than they actually are. Adjuvant! Online was designed to
be used in the adjuvant setting, but the majority of lung cancer patients never
have completely resected disease. Patients who were in good enough shape to
undergo at least a lobectomy are not your average patients with lung cancer.
DR RAVDIN: The average patients with lung cancer behave as if they’re three
to five years older than they actually are. Adjuvant! Online was designed to
be used in the adjuvant setting, but the majority of lung cancer patients never
have completely resected disease. Patients who were in good enough shape to
undergo at least a lobectomy are not your average patients with lung cancer.
Patients with Stage I disease have a ferocious mortality. Then you have
therapies — and there’s great debate as to whether it applies to those patients
— with 20 percent efficacy. That’s a substantial benefit. In breast cancer, we
would always treat without any question. In lung cancer, it’s less obvious to
the patient.
Track 6
 DR LOVE:
DR LOVE: Lowell, what are your thoughts on the cisplatin regimens for
treatment of non-small cell lung cancer (NSCLC) in community practice?
 DR HART: In the advanced disease setting, I believe cisplatin/docetaxel is
better than cisplatin/vinorelbine. I am wondering, does that have any bearing
on any of your decisions about the selection of adjuvant therapy?
DR HART: In the advanced disease setting, I believe cisplatin/docetaxel is
better than cisplatin/vinorelbine. I am wondering, does that have any bearing
on any of your decisions about the selection of adjuvant therapy?
 DR MILLER: Your overall impression is that of the oncology community.
In the paper evaluating cisplatin/docetaxel versus cisplatin/vinorelbine, the p-value for survival is 0.044 (Fossella 2003). Do I think that makes it reasonable
to extrapolate and use cisplatin/docetaxel in the adjuvant setting? Yes, I
believe it’s a reasonable thing to do. However, I don’t know that it’s any easier
when you administer it every three weeks as opposed to smaller, divided doses
of cisplatin/vinorelbine.
DR MILLER: Your overall impression is that of the oncology community.
In the paper evaluating cisplatin/docetaxel versus cisplatin/vinorelbine, the p-value for survival is 0.044 (Fossella 2003). Do I think that makes it reasonable
to extrapolate and use cisplatin/docetaxel in the adjuvant setting? Yes, I
believe it’s a reasonable thing to do. However, I don’t know that it’s any easier
when you administer it every three weeks as opposed to smaller, divided doses
of cisplatin/vinorelbine.
 DR LANGER: I have no personal experience with docetaxel/cisplatin in the adjuvant setting. A lot of my colleagues are using it because in advanced
disease it was noninferior to vinorelbine/cisplatin. I believe most of us would
interpret that it is at least as good, if not better.
DR LANGER: I have no personal experience with docetaxel/cisplatin in the adjuvant setting. A lot of my colleagues are using it because in advanced
disease it was noninferior to vinorelbine/cisplatin. I believe most of us would
interpret that it is at least as good, if not better.
Having said that, there are no Phase III data with that combination in the
adjuvant setting. All of the data are with vinorelbine/cisplatin or paclitaxel/carboplatin. So it’s all by extrapolation. I don’t think it’s going to be worse,
and I doubt it’s going to be significantly better.
The upcoming ECOG trial (ECOG-E1505; [3.2]) is going to feature
a “chemo du jour,” with a menu of options to pick from. I don’t feel we’re as
caught up in lung cancer as the breast cancer world is in comparing minute
changes in regimens. The presumption is that various platinum cocktails are
probably going to achieve the same result. We have to ask other questions,
particularly about the introduction of targeted agents.
Tracks 7-8
 DR LOVE:
DR LOVE: Corey, can you update us on the toxicity associated with
bevacizumab in patients with lung cancer and, from a safety perspective,
how you think bevacizumab is going to play out in the adjuvant setting in
lung cancer?
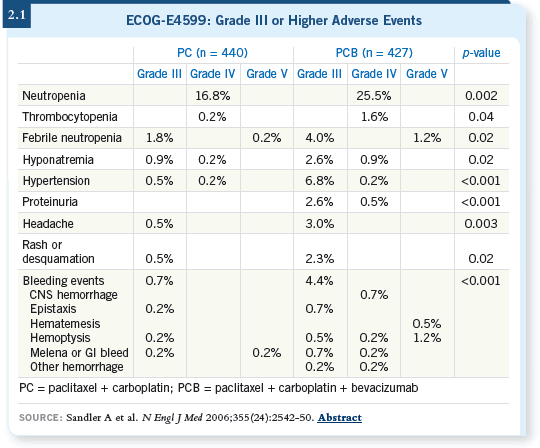
 DR LANGER: In ECOG-E4599, the addition of bevacizumab to paclitaxel/carboplatin demonstrated a two-month improvement in median overall
survival and about a six to eight percent improvement in one- and two-year
survival. It also showed more toxicity, particularly pulmonary hemorrhage
(Sandler 2006a; [2.1]).
DR LANGER: In ECOG-E4599, the addition of bevacizumab to paclitaxel/carboplatin demonstrated a two-month improvement in median overall
survival and about a six to eight percent improvement in one- and two-year
survival. It also showed more toxicity, particularly pulmonary hemorrhage
(Sandler 2006a; [2.1]).
In the bevacizumab/paclitaxel/carboplatin arm, 15 treatment-related deaths
occurred out of 305 patients. Not all were related to hemorrhage — some
were from neutropenic fever or other causes. In the control group, two treatment-related deaths occurred out of 344 patients. So, although we excluded
patients with squamous histology, brain metastases, ongoing thromboembolic
phenomena, anticoagulation use or antecedent hemoptysis, we still saw a
heightened treatment-related death rate (Sandler 2006a).
I believe many of those concerns are going to fall by the wayside in the adjuvant
trial. The tumors have been resected. By definition, these patients have no
residual tumor in the chest. Ideally, they should not have pulmonary hemorrhage.
Track 12
 DR LOVE:
DR LOVE: Do you think the cavitation and bleeding associated with
bevacizumab are related to its mechanism of action or to the response to
therapy?
 DR LANGER: Definitely the response. Many of the patients in whom we’ve
seen some of the more worrisome bleeding episodes have actually demonstrated tumor response. So it could be that these patients are, in fact, responding
too quickly. Whether it has something to do with the mechanism of action is
unclear to me. It’s conceivable that these folks may have more vascular tumors
and, therefore, we see more necrosis, but that’s purely speculative.
DR LANGER: Definitely the response. Many of the patients in whom we’ve
seen some of the more worrisome bleeding episodes have actually demonstrated tumor response. So it could be that these patients are, in fact, responding
too quickly. Whether it has something to do with the mechanism of action is
unclear to me. It’s conceivable that these folks may have more vascular tumors
and, therefore, we see more necrosis, but that’s purely speculative.
 DR LOVE: Vince, what do you think is happening with these patients?
DR LOVE: Vince, what do you think is happening with these patients?
 DR MILLER: Although the data have not yet been presented, it’s widely
known from the folks at ECOG that in a Phase II trial (ECOG-E3501) of
patients with small cell lung cancer, which had an interim analysis with 50
or 60 patients, there were essentially no Grade IV or V pulmonary events.
Those are centrally located tumors, which are often endobronchial and
change rapidly in response to chemotherapy. So I believe it’s something largely
peculiar to squamous cell histology.
DR MILLER: Although the data have not yet been presented, it’s widely
known from the folks at ECOG that in a Phase II trial (ECOG-E3501) of
patients with small cell lung cancer, which had an interim analysis with 50
or 60 patients, there were essentially no Grade IV or V pulmonary events.
Those are centrally located tumors, which are often endobronchial and
change rapidly in response to chemotherapy. So I believe it’s something largely
peculiar to squamous cell histology.
Select Publications

