
| Tracks 1-11 |
| Track 1 |
Introduction |
| Track 2 |
Development of novel TKIs in NSCLC |
| Track 3 |
Combination therapy with bevacizumab and erlotinib |
| Track 4 |
Predictors of response to the EGFR TKIs |
| Track 5 |
Phase II trial of carboplatin, nab paclitaxel and bevacizumab for patients with nonsquamous-cell NSCLC |
| Track 6 |
Secreted protein acidic and rich in cysteine (SPARC), caveolin-1 and enhanced delivery of nab paclitaxel |
|
| Track 7 |
Clinical trial results with MUC1 and MAGE vaccines in NSCLC |
| Track 8 |
Clinical research strategies to identify targets for chemotherapy and novel agents |
| Track 9 |
Evaluation of the novel anthracycline amrubicin in the treatment of SCLC |
| Track 10 |
Incorporation of bevacizumab into the treatment of NSCLC |
| Track 11 |
Lung cancer in smokers and nonsmokers: Implications for treatment approaches |
|
|
Select Excerpts from the Interview
Track 4
 DR LOVE:
DR LOVE: Can you summarize where we are right now in terms of
predictors of patient response to tyrosine kinase inhibitors, particularly
erlotinib?
 DR REYNOLDS: I believe there are a couple ways you can look at it. One is to
view it from a clinical physician standpoint and say female patients are more
likely to respond than male patients, Asians are more likely to respond than
non-Asians and nonsmokers are much more likely to respond than smokers. In
general, using these clinical factors can provide a good estimate of the likelihood
of response. That does not mean that a male smoker with squamous cell
carcinoma has no chance of benefit from erlotinib, but the likelihood of a
huge benefit is much less.
DR REYNOLDS: I believe there are a couple ways you can look at it. One is to
view it from a clinical physician standpoint and say female patients are more
likely to respond than male patients, Asians are more likely to respond than
non-Asians and nonsmokers are much more likely to respond than smokers. In
general, using these clinical factors can provide a good estimate of the likelihood
of response. That does not mean that a male smoker with squamous cell
carcinoma has no chance of benefit from erlotinib, but the likelihood of a
huge benefit is much less.
Another view is from the biology perspective, and a few ideas have been investigated. One is EGFR gene amplification. That work has been conducted
predominantly by Fred Hirsch at Colorado, and his team has shown fairly
compellingly that overexpression of the EGFR gene in tumors predicts
response and survival in lung cancer.
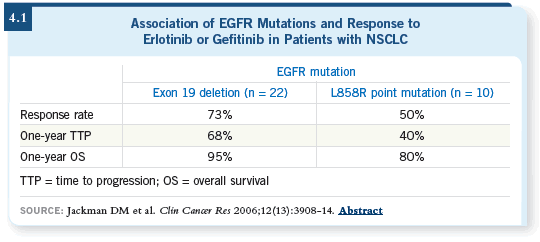
The other part of the biology equation has been EGFR mutations, with
research pioneered primarily by Tom Lynch at Harvard. He has been able to
show certain mutations, particularly an exon 19 deletion, for example, that
tend to predict good response to therapy (Jackman 2006; [4.1]).
Every clinician who has used erlotinib or gefitinib extensively has most likely
had one or two patients who have done incredibly well on the drug. I feel that
gene amplification is a better predictor of the group of patients that derives
meaningful benefit from the drug but not the spectacular home run we
occasionally see.
Tracks 5-6
 DR LOVE:
DR LOVE: Can you discuss the study you just reported evaluating bevacizumab,
nab paclitaxel and carboplatin?
 DR REYNOLDS: We conducted a single-arm Phase II trial evaluating the
combination of bevacizumab, nab paclitaxel and carboplatin in nonsquamous
NSCLC, and this trial was developed after the results of ECOG-E4599
became available (Sandler 2006). In fact, we were developing the trial without
bevacizumab and then thought the combination of nab paclitaxel and bevacizumab
offered promise for lung cancer patients.
DR REYNOLDS: We conducted a single-arm Phase II trial evaluating the
combination of bevacizumab, nab paclitaxel and carboplatin in nonsquamous
NSCLC, and this trial was developed after the results of ECOG-E4599
became available (Sandler 2006). In fact, we were developing the trial without
bevacizumab and then thought the combination of nab paclitaxel and bevacizumab
offered promise for lung cancer patients.
Rakesh Jain compellingly suggested with his research (Willett 2004) that
one of the major roles of bevacizumab in improving outcomes has to do with
improving drug delivery into tumors by changing tumor oncotic pressure and
normalizing vasculature. Compelling preclinical and early clinical data with
nab paclitaxel show that the formulation of nab paclitaxel does a better job
of tumor drug delivery, so we thought the combination of these two drugs
would potentially improve outcomes.
We embarked on a single-arm Phase II trial with entry criteria that were
fairly similar to those used in ECOG-E4599, and the results we have seen are
promising. Thus far with 50 patients, response rates are in the range of 30
percent, and we did not see any toxicity different from what we would expect
based on ECOG-E4599.
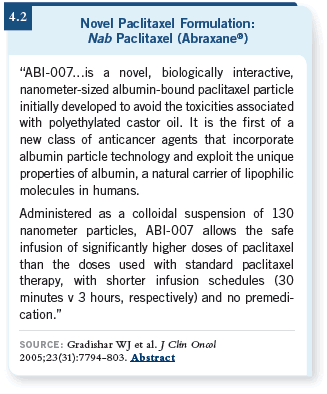
 DR LOVE: How much of
an advantage is the shorter
infusion time with nab paclitaxel
(4.2)?
DR LOVE: How much of
an advantage is the shorter
infusion time with nab paclitaxel
(4.2)?
 DR REYNOLDS: The shortened
time in the office is
significant, especially when
you are dealing with patients
who will only live about
another year.
DR REYNOLDS: The shortened
time in the office is
significant, especially when
you are dealing with patients
who will only live about
another year.
 DR LOVE: What is the next
step in terms of studying
nab paclitaxel?
DR LOVE: What is the next
step in terms of studying
nab paclitaxel?
 DR REYNOLDS: The current
strategy is to embark on a
Phase III trial. Whether that
trial will involve bevacizumab
is still a matter of
debate, although I believe the
study that will go forward
is standard paclitaxel with
carboplatin versus nab paclitaxel with carboplatin for advanced lung cancer treatment.
The possibility of a study in the adjuvant setting has also been discussed.
DR REYNOLDS: The current
strategy is to embark on a
Phase III trial. Whether that
trial will involve bevacizumab
is still a matter of
debate, although I believe the
study that will go forward
is standard paclitaxel with
carboplatin versus nab paclitaxel with carboplatin for advanced lung cancer treatment.
The possibility of a study in the adjuvant setting has also been discussed.
We have to be careful to ask questions that benefit our patients, and my
enthusiasm for using these engineered taxanes in general has to do with the
possibility of improving outcomes. Compelling data with at least a couple of
these drugs indicate better delivery into the tumor and therefore potentially
improved outcomes.
Select Publications

