 DR HERBST: Clearly, this patient is one
of the 10 percent or so of patients who
demonstrate response to these TKIs. I
would bet, if we looked at her tumor, we
would see either an EGFR mutation or
FISH overexpression. The concern is how
long this response will last.
DR HERBST: Clearly, this patient is one
of the 10 percent or so of patients who
demonstrate response to these TKIs. I
would bet, if we looked at her tumor, we
would see either an EGFR mutation or
FISH overexpression. The concern is how
long this response will last.
People develop resistance to these agents,
and she needs to be watched closely. I
would probably order a scan for her
every three or four months and think
about adding something else at the first
sign of progression.
 DR KIM: I am extremely puzzled by this
case. The liver toxicity humbles us. Erlotinib
was branded, originally, as a more
toxic version of gefitinib. Although we
know of other inherent differences, it is
puzzling to see similarly structured drugs
with the lower-dosed drug causing the
profound transaminitis and the higher-dosed
drug being exceptionally tolerable.
DR KIM: I am extremely puzzled by this
case. The liver toxicity humbles us. Erlotinib
was branded, originally, as a more
toxic version of gefitinib. Although we
know of other inherent differences, it is
puzzling to see similarly structured drugs
with the lower-dosed drug causing the
profound transaminitis and the higher-dosed
drug being exceptionally tolerable.
That makes this a very interesting case. It
teaches us a lesson that, indeed, these are
different drugs, and even though the structural
differences are minor, the distinction
is not all dose related.
 DR LOVE: Roy, what if she progressed
objectively on erlotinib and was still
clinically stable?
DR LOVE: Roy, what if she progressed
objectively on erlotinib and was still
clinically stable?
 DR HERBST: She’s received the EGFR
inhibitors as second-line therapy, so one
option if she were to progress would be to
use another chemotherapeutic agent.
DR HERBST: She’s received the EGFR
inhibitors as second-line therapy, so one
option if she were to progress would be to
use another chemotherapeutic agent.
The agents one would consider would be
pemetrexed or docetaxel (Hanna 2004),
although I’m partial to the combination of
bevacizumab and erlotinib (Herbst 2005a).
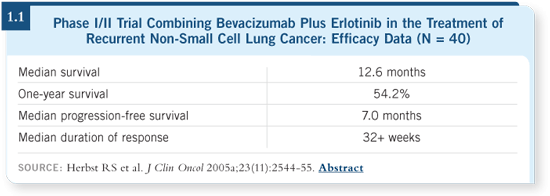
We recently published our Phase II experience
at MD Anderson and Vanderbilt, in
which that combination in second- or later-line
therapy had a time to progression close
to seven months, with a median survival
of more than a year (Herbst 2005a; [1.1]). Those data are currently being confirmed.
At ASCO this year we’ll hear about a large
multicenter Phase II randomized trial that
was conducted throughout the United
States.
An ongoing trial is evaluating bevacizumab
with erlotinib versus erlotinib alone in the
second-line metastatic setting: the BETA-2 lung trial.
Based on my experience, if no contraindications
were present — for example, disease
in the brain — I probably would consider
adding bevacizumab to the erlotinib if the
tumor progresses.
 DR LOVE: Are you more inclined to do this because she’s had such a great response
to erlotinib?
DR LOVE: Are you more inclined to do this because she’s had such a great response
to erlotinib?
 DR HERBST: Yes, I probably wouldn’t
recommend it off study for someone “cold.”
If a patient comes in to whom you want to
administer the combination of an EGFR
inhibitor and an angiogenesis inhibitor, I’m
a great proponent of clinical studies. But in
this case, when you already have a patient
who is responding to erlotinib, I think it
would be reasonable to consider adding the
angiogenesis inhibitor.
DR HERBST: Yes, I probably wouldn’t
recommend it off study for someone “cold.”
If a patient comes in to whom you want to
administer the combination of an EGFR
inhibitor and an angiogenesis inhibitor, I’m
a great proponent of clinical studies. But in
this case, when you already have a patient
who is responding to erlotinib, I think it
would be reasonable to consider adding the
angiogenesis inhibitor.
 DR SEIGEL: If we didn’t use bevacizumab
and we were going to start chemotherapy
with a taxane, would you still
continue the erlotinib, even if she objectively
progressed on it?
DR SEIGEL: If we didn’t use bevacizumab
and we were going to start chemotherapy
with a taxane, would you still
continue the erlotinib, even if she objectively
progressed on it?
 DR HERBST: That is a big question
that we all grapple with as we see patients
who benefit from the EGFR inhibitors.
This is a group for which adding chemotherapy
might provide a benefit. Certainly,
we know that the never-smokers treated
with chemotherapy and erlotinib had a
wonderful outcome, a median survival of
longer than 20 months, but it was a small
group of patients (Herbst 2005b).
DR HERBST: That is a big question
that we all grapple with as we see patients
who benefit from the EGFR inhibitors.
This is a group for which adding chemotherapy
might provide a benefit. Certainly,
we know that the never-smokers treated
with chemotherapy and erlotinib had a
wonderful outcome, a median survival of
longer than 20 months, but it was a small
group of patients (Herbst 2005b).
The problem here is that we might have
missed our window. At the point when
she’s starting to progress, you have to
assume that something has changed in her
EGFR axis. The drug might still provide
a benefit, but it’s not a benefit in terms of
apoptosis. I probably wouldn’t mix the two.
I would either add an anti-angiogenic agent
or look for clinical trials.
Select publications

