
Select Excerpts from the Discussion
Track 4
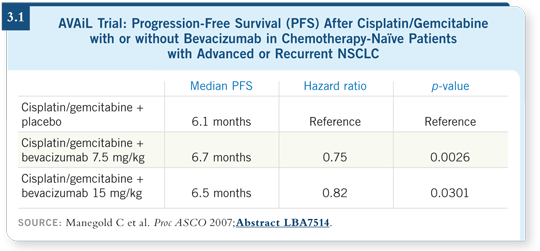
 DR KIM: The AVAiL trial was evaluating cisplatin/gemcitabine, with two
different doses of bevacizumab (versus placebo) — 7.5 mg/kg and 15 mg/kg
— which were grouped for the final analysis (Manegold 2007; [3.1]).
DR KIM: The AVAiL trial was evaluating cisplatin/gemcitabine, with two
different doses of bevacizumab (versus placebo) — 7.5 mg/kg and 15 mg/kg
— which were grouped for the final analysis (Manegold 2007; [3.1]).
The primary endpoint of the study was progression-free survival, and it was
not powered for overall survival.
The study was positive for the addition of bevacizumab at the 7.5-mg/kg and
15-mg/kg doses compared to placebo. I should note that 15 mg/kg is the
established dose of bevacizumab in NSCLC currently.
We knew everyone would start comparing the two doses of bevacizumab
— however, this trial was not powered to show that difference directly.
We don’t have overall survival data yet.
The importance of the AVAiL study is that it is the second positive Phase
III trial with bevacizumab in front-line NSCLC. It supports the use of
bevacizumab in the first-line setting with chemotherapy.
Obvious benefit was seen with both of the doses of bevacizumab — the
standard 15-mg/kg dose and the lower dose of 7.5 mg/kg.
 DR LYNCH: The good news from the AVAiL trial is that the study was
positive and both doses are safe. I believe it’s reasonable to continue to administer
15 mg/kg. The AVAiL trial also demonstrated that bevacizumab can be
given safely with a nonpaclitaxel-containing regimen.
DR LYNCH: The good news from the AVAiL trial is that the study was
positive and both doses are safe. I believe it’s reasonable to continue to administer
15 mg/kg. The AVAiL trial also demonstrated that bevacizumab can be
given safely with a nonpaclitaxel-containing regimen.
A slight increase was seen in the rate of hypertension with the 15-mg/kg dose
compared to 7.5 mg/kg.
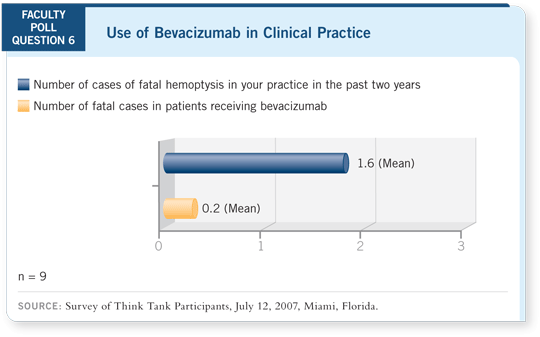
However, most importantly, the toxicities we’re most worried about —
hemoptysis and bleeding — were similar between the two arms (Manegold
2007). Safety-wise, people can feel comfortable with either dose of
bevacizumab.
 DR LOVE: Joan, one of the first questions that came up after this
presentation was, what about the dose of bevacizumab in the adjuvant study,
ECOG-E1505?
DR LOVE: Joan, one of the first questions that came up after this
presentation was, what about the dose of bevacizumab in the adjuvant study,
ECOG-E1505?
 DR SCHILLER: Because all of the data we have in terms of survival are with
the 15-mg/kg dose, that’s the dose we will use going forward.
DR SCHILLER: Because all of the data we have in terms of survival are with
the 15-mg/kg dose, that’s the dose we will use going forward.
Track 6
 DR LOVE:
DR LOVE: Alan, we are interested in your perception of the AVAiL trial
results because of your involvement with ECOG-E4599.
 DR SANDLER: : The AVAiL study provides more supportive evidence that
bevacizumab has activity in NSCLC when administered with chemotherapy,
in this case a cisplatin-based regimen (Manegold 2007). It was designed to
evaluate time to progression, and it met its endpoint. It was not designed
specifically to evaluate the two different doses.
DR SANDLER: : The AVAiL study provides more supportive evidence that
bevacizumab has activity in NSCLC when administered with chemotherapy,
in this case a cisplatin-based regimen (Manegold 2007). It was designed to
evaluate time to progression, and it met its endpoint. It was not designed
specifically to evaluate the two different doses.
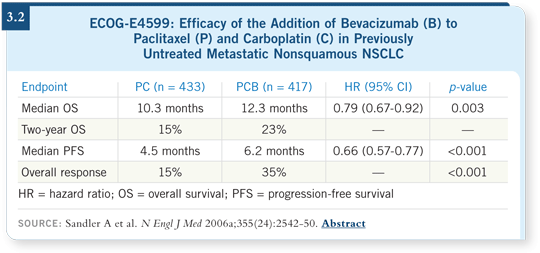
ECOG-E4599 is the only study that has shown a survival advantage, and the
dose of bevacizumab was 15 mg/kg (Sandler 2006a; [3.2]). That’s the dose
that has the best level of evidence.
The AVAiL trial also demonstrated that the toxicity with bevacizumab was
similar to what was seen in ECOG-E4599. The incidence of bleeding was
about the same, maybe a bit lower. Hypertension was roughly the same, or
perhaps a bit higher. Within the AVAiL trial, the incidence of some of the
noncritical toxicities was a little higher with the higher dose. For example,
proteinuria and hypertension may have been more dose related, but the
severe toxicities were roughly similar across both bevacizumab arms
(Manegold 2007).
Tracks 7, 10
 DR LOVE:
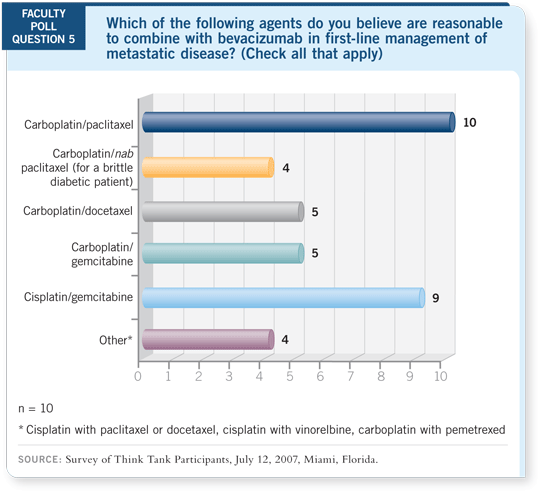
DR LOVE: Ed, which chemotherapy agents do you tend to combine with
bevacizumab in metastatic disease?
 DR KIM: I believe any one of these regimens with carboplatin or cisplatin is
reasonable with
DR KIM: I believe any one of these regimens with carboplatin or cisplatin is
reasonable with
15 mg/kg of bevacizumab. We have enough safety data. At our institution, we try to enroll in a study first. If that’s not possible, I wrote the
trial with carboplatin/docetaxel/bevacizumab, and that’s been my preference
off protocol. However, I believe either taxane is reasonable.
 DR LOVE: Vince, how are you approaching these questions off study — the
dose of bevacizumab and the choice of chemotherapeutic agent?
DR LOVE: Vince, how are you approaching these questions off study — the
dose of bevacizumab and the choice of chemotherapeutic agent?
 DR MILLER: I continue to use 15 mg/kg of bevacizumab, but I’ve homed
in more on a platinum/taxane doublet until we have survival data from the
AVAiL study.
DR MILLER: I continue to use 15 mg/kg of bevacizumab, but I’ve homed
in more on a platinum/taxane doublet until we have survival data from the
AVAiL study.
 DR GRECO: A purist would say that only paclitaxel and carboplatin should
be used. In my opinion, any of the chemotherapy regimens thought to be
equivalent in advanced NSCLC and that have Phase II safety data are
reasonable to use with bevacizumab.
DR GRECO: A purist would say that only paclitaxel and carboplatin should
be used. In my opinion, any of the chemotherapy regimens thought to be
equivalent in advanced NSCLC and that have Phase II safety data are
reasonable to use with bevacizumab.
Track 11
 DR LOVE:
DR LOVE: Do you believe bevacizumab-related hemoptysis is associated
with tumor response?
 DR LYNCH: I would say yes — it is associated with a response. The only
problem is that some people bleed in the first or second cycle.
DR LYNCH: I would say yes — it is associated with a response. The only
problem is that some people bleed in the first or second cycle.
 DR LOVE: Let’s say you see a patient who starts to have a rapid response that is
cavitary. How will you react?
DR LOVE: Let’s say you see a patient who starts to have a rapid response that is
cavitary. How will you react?
 DR LYNCH: I recently received an email from a colleague who showed me a beautiful response to carboplatin/paclitaxel/bevacizumab. It had become
completely cavitary. He asked me, “Tom, what do I do now?” I said, “Keep
going. That’s what you’re aiming for.”
DR LYNCH: I recently received an email from a colleague who showed me a beautiful response to carboplatin/paclitaxel/bevacizumab. It had become
completely cavitary. He asked me, “Tom, what do I do now?” I said, “Keep
going. That’s what you’re aiming for.”
This doctor consulted with three other doctors, took the patient off the study,
radiated the lung and put the patient on maintenance bevacizumab afterward.
The presence of the cavitation led this doctor to use radiation therapy. I’m
curious how other people would handle that large cavitary response.
 DR CURRAN: Intuitively it makes sense that it could work because radiation
therapy has a hemostatic effect. There are even trials now in which a small dose
of radiation is administered prior to the bevacizumab for patients thought to
be at high risk — those with squamous histology, central disease, a history of
hemoptysis or some other endobronchial disease.
DR CURRAN: Intuitively it makes sense that it could work because radiation
therapy has a hemostatic effect. There are even trials now in which a small dose
of radiation is administered prior to the bevacizumab for patients thought to
be at high risk — those with squamous histology, central disease, a history of
hemoptysis or some other endobronchial disease.
 DR SCHILLER: In ECOG-E4599, we retrospectively evaluated any factors that
would predict for fatal bleeds. The only one that stood out was pretreatment
cavitation (Sandler 2006b).
DR SCHILLER: In ECOG-E4599, we retrospectively evaluated any factors that
would predict for fatal bleeds. The only one that stood out was pretreatment
cavitation (Sandler 2006b).
 DR SANDLER: : Remember, the numbers are small. We combined the Phase II
study with ECOG-E4599, and the overall number of significant pulmonary
hemorrhages was relatively small (Sandler 2006b).
DR SANDLER: : Remember, the numbers are small. We combined the Phase II
study with ECOG-E4599, and the overall number of significant pulmonary
hemorrhages was relatively small (Sandler 2006b).
Track 12
 DR HANNA: I believe the central location of the tumor is a big risk factor. I
do not administer bevacizumab to patients with Stage III disease who have
central cavitary lesions. At this point, even if we radiate these tumors ahead of
time, we don’t know if it will make a difference.
DR HANNA: I believe the central location of the tumor is a big risk factor. I
do not administer bevacizumab to patients with Stage III disease who have
central cavitary lesions. At this point, even if we radiate these tumors ahead of
time, we don’t know if it will make a difference.
If it’s mediastinal lymphadenopathy, location doesn’t worry me. If the tumor is
bulky and located next to the pulmonary artery, that worries me.
 DR SANDLER: : Looking back at the clinical studies, location has never been an
issue. Is it the proximity to a vessel or bronchus that might be more important
in terms of causing bleeding and hemoptysis? None of that has been borne
out. You’re nervous when it’s central, but we forge ahead.
DR SANDLER: : Looking back at the clinical studies, location has never been an
issue. Is it the proximity to a vessel or bronchus that might be more important
in terms of causing bleeding and hemoptysis? None of that has been borne
out. You’re nervous when it’s central, but we forge ahead.
 DR LYNCH: The AVAiL study did not find any relationship to location.
I believe that centrality won’t be a big concern.
DR LYNCH: The AVAiL study did not find any relationship to location.
I believe that centrality won’t be a big concern.
Tracks 28, 31
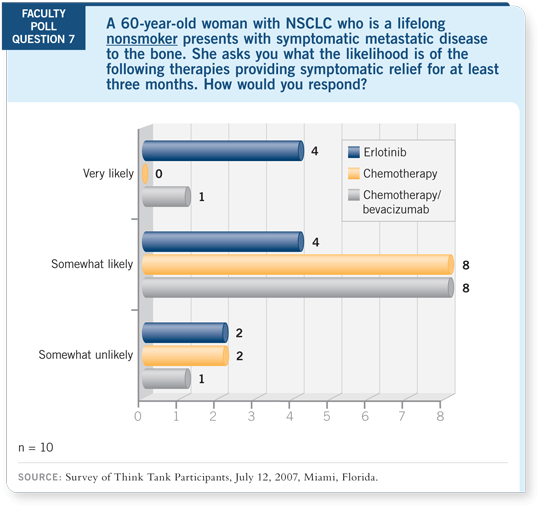
 DR LOVE:
DR LOVE: If you have a patient who is a lifelong nonsmoker and has
symptomatic metastatic disease, what is the likelihood that she’s going to
derive pain relief from antitumor therapy? Most of you thought she would
be likely to obtain pain relief from erlotinib but not as likely with chemotherapy.
Vince, does that mean that in these situations you would consider
erlotinib as first-line therapy?
 DR MILLER: I tend to use erlotinib more either as first-line or third-line
therapy. I’m driven by either the mutation status or clinical factors to incorporate
erlotinib into therapy early on. If patients have favorable profiles (ie,
a mutation that confers a 75 percent positive predictive value for response),
they live a long time. It’s simply a matter of time until we establish a survival
benefit for patients with mutations, but we need the trials to be completed.
DR MILLER: I tend to use erlotinib more either as first-line or third-line
therapy. I’m driven by either the mutation status or clinical factors to incorporate
erlotinib into therapy early on. If patients have favorable profiles (ie,
a mutation that confers a 75 percent positive predictive value for response),
they live a long time. It’s simply a matter of time until we establish a survival
benefit for patients with mutations, but we need the trials to be completed.
 DR KIM: We don’t perform mutation testing on everyone, but when we see
patients with these clinical factors — never smokers or adenocarcinoma with
BAC features — I use the standard option of chemotherapy/bevacizumab. The
second aspect would be to consider the nonstandard therapy — erlotinib.
DR KIM: We don’t perform mutation testing on everyone, but when we see
patients with these clinical factors — never smokers or adenocarcinoma with
BAC features — I use the standard option of chemotherapy/bevacizumab. The
second aspect would be to consider the nonstandard therapy — erlotinib.
 DR GRECO: Patients need to be selected out. For those presenting in the first-line
setting who are nonsmokers, my choice would be erlotinib.
DR GRECO: Patients need to be selected out. For those presenting in the first-line
setting who are nonsmokers, my choice would be erlotinib.
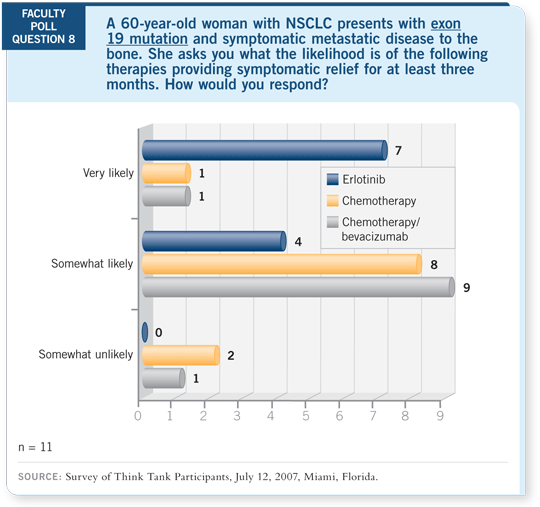
With the mutations, the rate of benefit is even higher. We can identify a
group of patients, clinically, who are likely to respond. I won’t use first-line
erlotinib with the majority of patients who have FISH-negative disease, have
no mutations or have been smoking heavily and have RAS mutations.
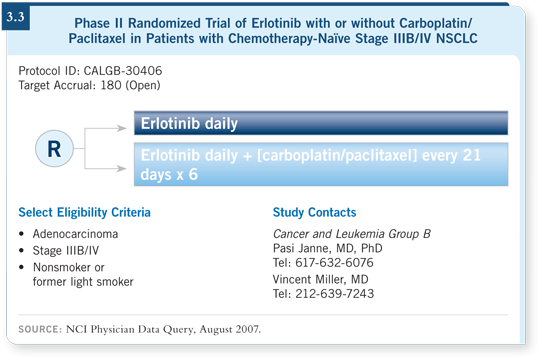
 DR SOCINSKI: I believe erlotinib is an important drug for never smokers. If they
don’t want to enroll on CALGB-30406 (3.3), I tend to treat never smokers with
four cycles of chemotherapy and bevacizumab followed by immediate erlotinib.
DR SOCINSKI: I believe erlotinib is an important drug for never smokers. If they
don’t want to enroll on CALGB-30406 (3.3), I tend to treat never smokers with
four cycles of chemotherapy and bevacizumab followed by immediate erlotinib.
Track 43
 DR LOVE:
DR LOVE: What is the potential role of nanoparticle albumin-bound (
nab)
paclitaxel in lung cancer treatment?
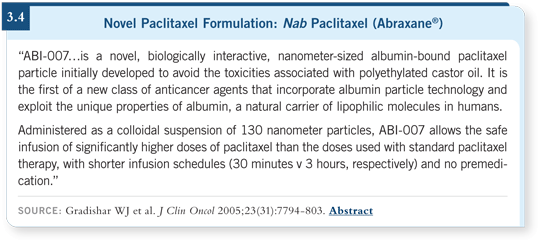
 DR GRECO: It has a different type of neurotoxicity that usually abates. It’s less
toxic and easier to use than the standard paclitaxel formulation (3.4), and the
breast cancer data are impressive. We don’t have definitive data in lung cancer
yet, but there will be a Phase III trial comparing nab paclitaxel to standard
paclitaxel. This agent may not be superior, but the toxicity advantages could
be important.
DR GRECO: It has a different type of neurotoxicity that usually abates. It’s less
toxic and easier to use than the standard paclitaxel formulation (3.4), and the
breast cancer data are impressive. We don’t have definitive data in lung cancer
yet, but there will be a Phase III trial comparing nab paclitaxel to standard
paclitaxel. This agent may not be superior, but the toxicity advantages could
be important.
 DR LOVE: If it turns out that the efficacy is the same but there’s less neurotoxicity
than with Cremophor-based paclitaxel, would that be enough for you?
DR LOVE: If it turns out that the efficacy is the same but there’s less neurotoxicity
than with Cremophor-based paclitaxel, would that be enough for you?
 DR GRECO: It would be enough for me. It’s already on the market and used
in breast cancer. I believe there are a number of circumstances in which less
neurotoxicity — for alcoholics, diabetics and others — is an advantage.
DR GRECO: It would be enough for me. It’s already on the market and used
in breast cancer. I believe there are a number of circumstances in which less
neurotoxicity — for alcoholics, diabetics and others — is an advantage.
Track 45
 DR LOVE:
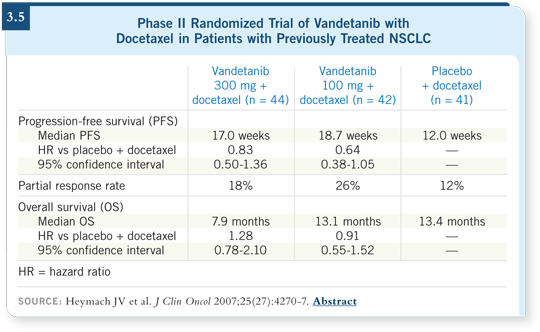
DR LOVE: Vince, can you discuss where we are with vandetanib
(ZD6474; [3.5])?
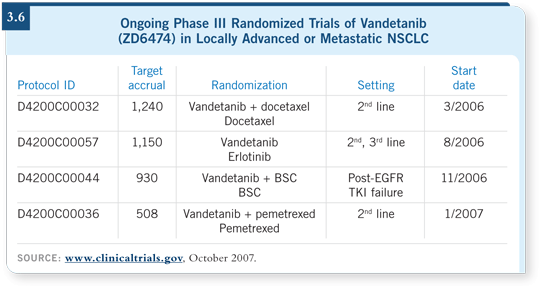
 DR MILLER: There are Phase III trials searching for benefits (3.6). It’s a
biologically active drug. In patients who have failed erlotinib, one trial is
comparing vandetanib to supportive care. Another trial is comparing it
to erlotinib after progression on chemotherapy. A third trial is evaluating
docetaxel with vandetanib as a second-line regimen versus docetaxel alone.
DR MILLER: There are Phase III trials searching for benefits (3.6). It’s a
biologically active drug. In patients who have failed erlotinib, one trial is
comparing vandetanib to supportive care. Another trial is comparing it
to erlotinib after progression on chemotherapy. A third trial is evaluating
docetaxel with vandetanib as a second-line regimen versus docetaxel alone.
 DR LOVE: If you had to guess which strategy would be the most effective
with vandetanib, what would you hypothesize?
DR LOVE: If you had to guess which strategy would be the most effective
with vandetanib, what would you hypothesize?
 DR LYNCH: I believe the placebo-controlled trials will most likely be positive.
However, we’ve made little progress in standard second-line therapy for lung
cancer, so I’d love to see the docetaxel combination study show benefit.
DR LYNCH: I believe the placebo-controlled trials will most likely be positive.
However, we’ve made little progress in standard second-line therapy for lung
cancer, so I’d love to see the docetaxel combination study show benefit.
Select Publications

