
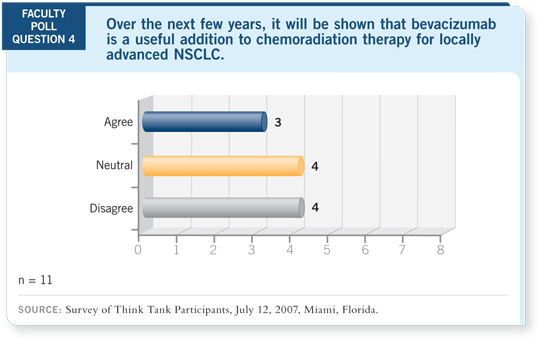
Select Excerpts from the Discussion
Track 37
 DR LOVE:
DR LOVE: Wally, can you discuss the clinical trial strategies incorporating
bevacizumab with chemoradiation therapy for Stage III disease?
 DR CURRAN: In SWOG-S0533, bevacizumab is introduced to a SWOG-S9504
core concurrently and after chemoradiation therapy either on day
one or 15 (2.1). The patients are stratified by risk for hemoptysis, based on
squamous-cell histology, history of hemoptysis and evidence of bulky disease.
Mark Socinski has an investigator-initiated trial also evaluating bevacizumab
with chemoradiation therapy.
DR CURRAN: In SWOG-S0533, bevacizumab is introduced to a SWOG-S9504
core concurrently and after chemoradiation therapy either on day
one or 15 (2.1). The patients are stratified by risk for hemoptysis, based on
squamous-cell histology, history of hemoptysis and evidence of bulky disease.
Mark Socinski has an investigator-initiated trial also evaluating bevacizumab
with chemoradiation therapy.
Preclinical data suggest that VEGF inhibition can sensitize tumor cells to
radiation therapy and chemoradiation therapy ( Jain 2001). We have to be
aware of competing toxicity — the greatest risk is of a tremendous antitumor
response that may result in a catastrophic local effect.
 DR LOVE: I didn’t realize that, historically, when only chemoradiation therapy was used, you initially saw tracheoesophageal (TE) fistulas because of tumor
response.
DR LOVE: I didn’t realize that, historically, when only chemoradiation therapy was used, you initially saw tracheoesophageal (TE) fistulas because of tumor
response.

 DR CURRAN: It even existed in the radiation therapy-alone era. My elders
taught me that if you have patients with esophageal or tracheal involvement,
use a lower radiation dose per fraction so as not to have such a rapid response
as to develop a fatal TE fistula. We need to figure out how to conduct these
new trials where we integrate three modalities providing us with a response.
DR CURRAN: It even existed in the radiation therapy-alone era. My elders
taught me that if you have patients with esophageal or tracheal involvement,
use a lower radiation dose per fraction so as not to have such a rapid response
as to develop a fatal TE fistula. We need to figure out how to conduct these
new trials where we integrate three modalities providing us with a response.
Tracks 39-42
 DR LOVE:
DR LOVE: Nasser, what’s your view of the clinical implications of the
HOG trial you presented at ASCO this year (Hanna 2007; [2.2])?
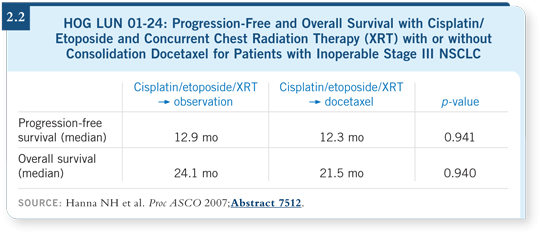
 DR HANNA: The important, larger picture with HOG LUN 01-24 is that we
previously had no randomized trial to support consolidation docetaxel, and now
our current randomized trial does not add support to its use (Hanna 2007).
DR HANNA: The important, larger picture with HOG LUN 01-24 is that we
previously had no randomized trial to support consolidation docetaxel, and now
our current randomized trial does not add support to its use (Hanna 2007).
 DR GRECO: I agree with Nasser that we really had no randomized data to
support what I call the “SWOG factor,” which may have inhibited our understanding
of how to treat unresectable Stage III disease, because more and more
patients were receiving that therapy in an ad hoc fashion, without the Phase
III data. HOG LUN 01-24 suggests that the majority of patients don’t benefit
from that approach, although some still may.
DR GRECO: I agree with Nasser that we really had no randomized data to
support what I call the “SWOG factor,” which may have inhibited our understanding
of how to treat unresectable Stage III disease, because more and more
patients were receiving that therapy in an ad hoc fashion, without the Phase
III data. HOG LUN 01-24 suggests that the majority of patients don’t benefit
from that approach, although some still may.
 DR LOVE: Corey, does HOG LUN 01-24 essentially end the use of
consolidation therapy?
DR LOVE: Corey, does HOG LUN 01-24 essentially end the use of
consolidation therapy?
 DR LANGER: I don’t believe consolidation therapy is dead. We need to
evaluate other drugs — preferably other targeted agents, particularly if we
figure out through molecular correlative studies who is more likely to respond
to the drugs. The blind shotgun approach probably doesn’t work here.
DR LANGER: I don’t believe consolidation therapy is dead. We need to
evaluate other drugs — preferably other targeted agents, particularly if we
figure out through molecular correlative studies who is more likely to respond
to the drugs. The blind shotgun approach probably doesn’t work here.
 DR LOVE: What are some of the innovative concepts you believe are worth
pursuing for patients with Stage III disease, Nasser?
DR LOVE: What are some of the innovative concepts you believe are worth
pursuing for patients with Stage III disease, Nasser?
 DR HANNA: Whether the anti-angiogenics will work well with chemoradiation
therapy is still not known. The bottom line is that the biologics must be
used in a targeted patient population. It is not one size fits all. We may identify
a subset of patients who should receive targeted agents, but the majority may
not benefit, except for anti-angiogenics, which could be the exception.
DR HANNA: Whether the anti-angiogenics will work well with chemoradiation
therapy is still not known. The bottom line is that the biologics must be
used in a targeted patient population. It is not one size fits all. We may identify
a subset of patients who should receive targeted agents, but the majority may
not benefit, except for anti-angiogenics, which could be the exception.
Select Publications

