
 |
|||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the current treatment options for patients
with advanced NSCLC?
DR LOVE: Would you discuss the current treatment options for patients
with advanced NSCLC?
![]() DR SANDLER: We have two positive studies evaluating regimens for
metastatic NSCLC in somewhat similar groups of patients (Manegold 2008;
Sandler 2006), and cisplatin/vinorelbine with cetuximab is now another
option (Pirker 2008; [2.1, page 8]). However, three issues need to be addressed
with cetuximab. The dermatologic reaction is not a life-threatening toxicity, but it is one that certain patients find difficult to live with. Another issue is
the inconvenience of a weekly injection.
DR SANDLER: We have two positive studies evaluating regimens for
metastatic NSCLC in somewhat similar groups of patients (Manegold 2008;
Sandler 2006), and cisplatin/vinorelbine with cetuximab is now another
option (Pirker 2008; [2.1, page 8]). However, three issues need to be addressed
with cetuximab. The dermatologic reaction is not a life-threatening toxicity, but it is one that certain patients find difficult to live with. Another issue is
the inconvenience of a weekly injection.
Finally, in certain parts of the country, an increased risk of anaphylactic reactions to cetuximab exists, although a recent New England Journal of Medicine article reported on the prospect of identifying patients who may be at risk for anaphylactic reactions (Chung 2008).
Tracks 2-4
![]() DR LOVE: What data sets have been reported in the past year addressing
chemotherapy in combination with bevacizumab?
DR LOVE: What data sets have been reported in the past year addressing
chemotherapy in combination with bevacizumab?
![]() DR SANDLER: A subset analysis of the ECOG-E4599 trial evaluated outcomes
for the elderly population because one fourth of the patients on the trial were
older than age 70.
DR SANDLER: A subset analysis of the ECOG-E4599 trial evaluated outcomes
for the elderly population because one fourth of the patients on the trial were
older than age 70.
A little more toxicity occurred in these older patients, which is not unexpected, but it was manageable. The response rate was better for patients treated with bevacizumab, and progression-free survival trended upward with a p-value close to 0.06 (Ramalingam 2008).
![]() DR LOVE: What do we know about bevacizumab-associated hemoptysis?
DR LOVE: What do we know about bevacizumab-associated hemoptysis?
![]() DR SANDLER: We learned in the Phase II study (AVF0757g) that patients
with squamous cell histology should not receive bevacizumab because of the
risk of hemoptysis. We’ve gone back and evaluated data from various studies,
including E4599, and only baseline cavitation emerged as a potential risk factor
(Sandler 2008).
DR SANDLER: We learned in the Phase II study (AVF0757g) that patients
with squamous cell histology should not receive bevacizumab because of the
risk of hemoptysis. We’ve gone back and evaluated data from various studies,
including E4599, and only baseline cavitation emerged as a potential risk factor
(Sandler 2008).
Interestingly, tumor size and location have not panned out as risk factors. Location may well be part of the squamous cell histology, but it’s difficult to tease the two apart.
![]() DR LOVE: Can you discuss the results of the AVAiL study?
DR LOVE: Can you discuss the results of the AVAiL study?
![]() DR SANDLER: The AVAiL study was the European counterpart of ECOG-E4599.
It used a three-arm design to evaluate cisplatin/gemcitabine with or
without bevacizumab at two different doses, 7.5 mg/kg and 15 mg/kg.
DR SANDLER: The AVAiL study was the European counterpart of ECOG-E4599.
It used a three-arm design to evaluate cisplatin/gemcitabine with or
without bevacizumab at two different doses, 7.5 mg/kg and 15 mg/kg.
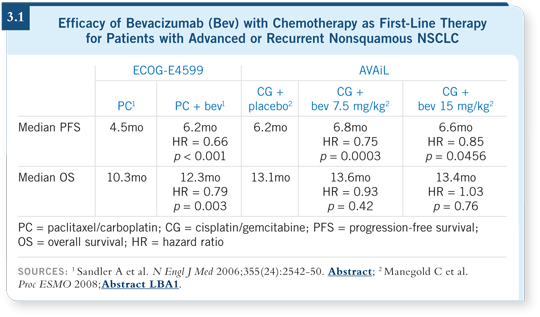
Response rate and progression-free survival endpoints were met in both the bevacizumab arms. Bevacizumab did not add significant benefit to survival, although overall survival was good in all three arms (Manegold 2008; [3.1]).
![]() DR LOVE: If someone were to say to you, “Why not use the lower dose of
bevacizumab,” how would you respond?
DR LOVE: If someone were to say to you, “Why not use the lower dose of
bevacizumab,” how would you respond?
![]() DR SANDLER: The AVAiL study is compelling. The hazard ratio for progression-free survival was 0.75 with the low dose and about 0.8 with the higher
dose, but E4599 is the only study to date that shows a survival advantage,
which is with the 15-mg/kg dose (3.1), so I am still administering that dose.
DR SANDLER: The AVAiL study is compelling. The hazard ratio for progression-free survival was 0.75 with the low dose and about 0.8 with the higher
dose, but E4599 is the only study to date that shows a survival advantage,
which is with the 15-mg/kg dose (3.1), so I am still administering that dose.
Track 9
![]() DR LOVE: What do we know about the multikinase inhibitor vandetanib?
DR LOVE: What do we know about the multikinase inhibitor vandetanib?
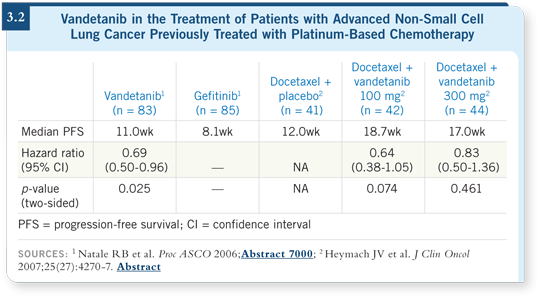
![]() DR SANDLER: Vandetanib inhibits both VEGF and EGFR to different
degrees. The low dose is more of a VEGF inhibitor, and the higher dose is
more of a combination of VEGF and EGFR inhibition.
DR SANDLER: Vandetanib inhibits both VEGF and EGFR to different
degrees. The low dose is more of a VEGF inhibitor, and the higher dose is
more of a combination of VEGF and EGFR inhibition.
As a single agent, the higher dose may be better, but in combination with chemotherapy, the lower dose may be preferable because of the potential antagonism between an EGFR agent and chemotherapy. Phase II studies suggest that the addition of vandetanib to either docetaxel or gefitinib may provide benefit (3.2).
Track 10
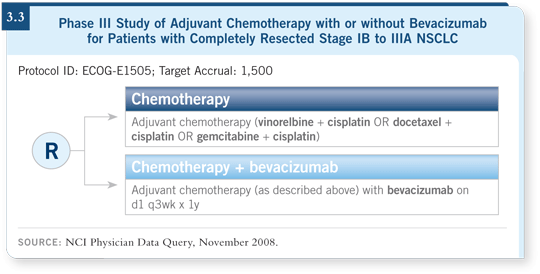
![]() DR LOVE: Could you provide an update on the adjuvant ECOG-E1505
trial evaluating chemotherapy with or without bevacizumab (3.3)?
DR LOVE: Could you provide an update on the adjuvant ECOG-E1505
trial evaluating chemotherapy with or without bevacizumab (3.3)?
![]() DR SANDLER: This study is evaluating cisplatin-based chemotherapy with or
without a year of bevacizumab in patients with tumors of all histologies. The
study has a target accrual of 1,500 patients with survival as the endpoint. The
study is not accruing nearly as fast as it should, with only about 250 patients
enrolled.
DR SANDLER: This study is evaluating cisplatin-based chemotherapy with or
without a year of bevacizumab in patients with tumors of all histologies. The
study has a target accrual of 1,500 patients with survival as the endpoint. The
study is not accruing nearly as fast as it should, with only about 250 patients
enrolled.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
David Jablons, MD
- Select publications
Gregory J Riely, MD, PhD
- Select publications
Alan B Sandler, MD
- Select publications
David S Ettinger, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity