
 |
|||||||

| Tracks 1-7 | ||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you comment on tumor tissue type and treatment
selection in NSCLC?
DR LOVE: Would you comment on tumor tissue type and treatment
selection in NSCLC?
![]() DR ETTINGER: Practice-changing data have emerged regarding the use of
histology to predict the effectiveness of different therapeutic agents.
DR ETTINGER: Practice-changing data have emerged regarding the use of
histology to predict the effectiveness of different therapeutic agents.
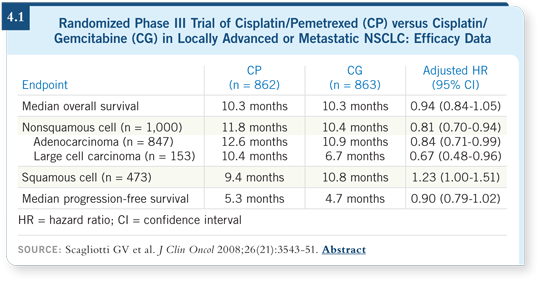
Dr Scagliotti recently published data in the Journal of Clinical Oncology from a study of gemcitabine/cisplatin versus pemetrexed/cisplatin in chemotherapy-naïve patients with advanced stage NSCLC, which demonstrated that patients with squamous cell histology had better outcomes with gemcitabine/cisplatin and those with adenocarcinoma fared better with pemetrexed/cisplatin (Scagliotti 2008; [4.1]).
As another example, Karp and colleagues presented a study at ASCO evaluating paclitaxel and carboplatin with CP-751,871 — a monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-IR) — in patients with advanced, treatment-naïve NSCLC (Karp 2008). The highest levels of IGF1 occur in squamous cell NSCLC. In patients with squamous cell tumors, 11 out of 14 patients responded, or 78 percent, whereas 57 percent of patients with adenocarcinomas demonstrated responses.
Track 3
![]() DR LOVE: What are your thoughts about ECOG-E1505 and the incorporation
of bevacizumab in the adjuvant setting?
DR LOVE: What are your thoughts about ECOG-E1505 and the incorporation
of bevacizumab in the adjuvant setting?
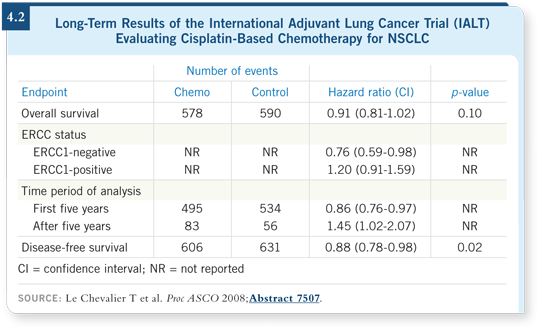
![]() DR ETTINGER: ECOG-E1505 is a great study because we haven’t seen
advances in adjuvant therapy for a while. In fact, at ASCO this year, Le
Chevalier presented the eight-year follow-up data for the IALT study and
showed that the survival advantage was no longer evident for chemotherapy
(Le Chevalier 2008; [4.2]). Interestingly, ERCC1 status remained predictive
for survival benefit from adjuvant chemotherapy (4.2).
DR ETTINGER: ECOG-E1505 is a great study because we haven’t seen
advances in adjuvant therapy for a while. In fact, at ASCO this year, Le
Chevalier presented the eight-year follow-up data for the IALT study and
showed that the survival advantage was no longer evident for chemotherapy
(Le Chevalier 2008; [4.2]). Interestingly, ERCC1 status remained predictive
for survival benefit from adjuvant chemotherapy (4.2).
![]() DR LOVE: How do you approach selection of adjuvant chemotherapy off
study?
DR LOVE: How do you approach selection of adjuvant chemotherapy off
study?
![]() DR ETTINGER: I use cisplatin paired with gemcitabine. If patients are intolerant
of cisplatin, I use carboplatin. The bigger issue is where pemetrexed will
fit in when it’s approved, especially for patients with adenocarcinomas.
DR ETTINGER: I use cisplatin paired with gemcitabine. If patients are intolerant
of cisplatin, I use carboplatin. The bigger issue is where pemetrexed will
fit in when it’s approved, especially for patients with adenocarcinomas.
Tracks 4-5
![]() DR LOVE: Can you discuss new research approaches to locally advanced
disease, particularly chemoradiation therapy and maintenance therapy?
DR LOVE: Can you discuss new research approaches to locally advanced
disease, particularly chemoradiation therapy and maintenance therapy?
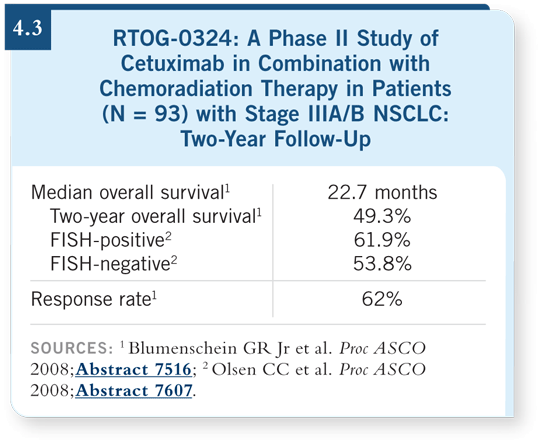
![]() DR ETTINGER: A study
presented by Blumenschein
and colleagues at ASCO
revealed a median survival
of 22.7 months with concurrent
chemoradiation therapy
and cetuximab, which is the
longest survival observed
in RTOG trials with Stage
III NSCLC (Blumenschein
2008; [4.3]). Sequential
chemoradiation therapy is
associated with a 14-month
median survival, and with
concurrent chemoradiation
therapy it’s about 17 months.
DR ETTINGER: A study
presented by Blumenschein
and colleagues at ASCO
revealed a median survival
of 22.7 months with concurrent
chemoradiation therapy
and cetuximab, which is the
longest survival observed
in RTOG trials with Stage
III NSCLC (Blumenschein
2008; [4.3]). Sequential
chemoradiation therapy is
associated with a 14-month
median survival, and with
concurrent chemoradiation
therapy it’s about 17 months.
The Intergroup study (RTOG-0617) of concurrent chemoradiation therapy, comparing two different doses of radiation therapy — 74 and 60 Gray — has added cetuximab. Based on our experience with cetuximab and chemotherapy (Vermorken 2008) combined with radiation therapy (Bonner 2006) in head and neck malignancies, we expect that cetuximab will be effective.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
David Jablons, MD
- Select publications
Gregory J Riely, MD, PhD
- Select publications
Alan B Sandler, MD
- Select publications
David S Ettinger, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity