
 |
|||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 3
![]() DR LOVE: What are your thoughts about the ECOG-E1505 adjuvant
study evaluating bevacizumab?
DR LOVE: What are your thoughts about the ECOG-E1505 adjuvant
study evaluating bevacizumab?
![]() DR HANNA: ECOG-E1505 is a randomized Phase III study that will treat
patients with resected, Stage IB to IIIA NSCLC. Patients will receive one of
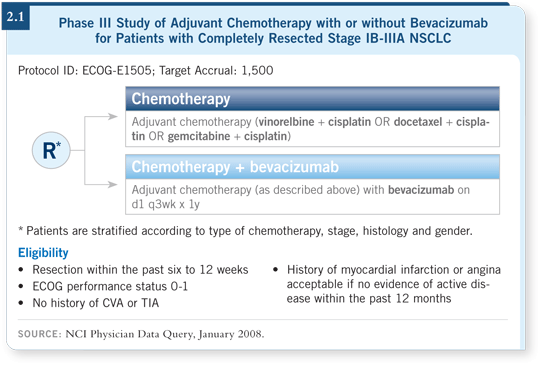
three cisplatin-based chemotherapy regimens — cisplatin/gemcitabine, cisplatin/docetaxel or cisplatin/vinorelbine — with or without bevacizumab (2.1).
DR HANNA: ECOG-E1505 is a randomized Phase III study that will treat
patients with resected, Stage IB to IIIA NSCLC. Patients will receive one of
three cisplatin-based chemotherapy regimens — cisplatin/gemcitabine, cisplatin/docetaxel or cisplatin/vinorelbine — with or without bevacizumab (2.1).
![]() DR LOVE: Currently, in your own practice outside of a protocol setting, how
are you approaching the selection of chemotherapy?
DR LOVE: Currently, in your own practice outside of a protocol setting, how
are you approaching the selection of chemotherapy?
![]() DR HANNA: I use cisplatin-based therapy unless there is a contraindication
such as modest renal insufficiency, in which case I administer carboplatin.
I believe little difference exists between the two agents, which may be
supported by early data from a European neoadjuvant trial presented at ASCO
this year (Milleron 2007).
DR HANNA: I use cisplatin-based therapy unless there is a contraindication
such as modest renal insufficiency, in which case I administer carboplatin.
I believe little difference exists between the two agents, which may be
supported by early data from a European neoadjuvant trial presented at ASCO
this year (Milleron 2007).
![]() DR LOVE: Which agent do you generally combine with cisplatin in the
adjuvant setting?
DR LOVE: Which agent do you generally combine with cisplatin in the
adjuvant setting?
![]() DR HANNA: I generally use docetaxel. The majority of data we have from
the adjuvant setting are with cisplatin/vinorelbine. However, you want to use
your best regimens from the metastatic setting in the adjuvant setting. Trials
have been conducted comparing cisplatin/docetaxel to cisplatin/vinorelbine
(Fossella 2003; Douillard 2005; [3.1]) or single-agent docetaxel to single-agent
vinorelbine, in which docetaxel was a more active and effective agent (Fossella
2000; Kudoh 2006). That is why I use cisplatin/docetaxel.
DR HANNA: I generally use docetaxel. The majority of data we have from
the adjuvant setting are with cisplatin/vinorelbine. However, you want to use
your best regimens from the metastatic setting in the adjuvant setting. Trials
have been conducted comparing cisplatin/docetaxel to cisplatin/vinorelbine
(Fossella 2003; Douillard 2005; [3.1]) or single-agent docetaxel to single-agent
vinorelbine, in which docetaxel was a more active and effective agent (Fossella
2000; Kudoh 2006). That is why I use cisplatin/docetaxel.
Track 9
![]() DR LOVE: Can you discuss the Hoosier Oncology Group (HOG) trial
data you presented at ASCO 2007?
DR LOVE: Can you discuss the Hoosier Oncology Group (HOG) trial
data you presented at ASCO 2007?
![]() DR HANNA: In 2003, SWOG published results from their Phase II trial,
S9504, which included 83 patients with Stage IIIB disease who were treated
with two cycles of cisplatin/etoposide concurrent with 61 Gray of radiation
followed by three cycles of docetaxel. The median survival was 26 months
(Gandara 2003, 2006). This population should have had a median survival of
about 13 months. They had a five-year survival of 29 percent. Historically,
that group should have had a five-year survival of five, seven or eight percent.
DR HANNA: In 2003, SWOG published results from their Phase II trial,
S9504, which included 83 patients with Stage IIIB disease who were treated
with two cycles of cisplatin/etoposide concurrent with 61 Gray of radiation
followed by three cycles of docetaxel. The median survival was 26 months
(Gandara 2003, 2006). This population should have had a median survival of
about 13 months. They had a five-year survival of 29 percent. Historically,
that group should have had a five-year survival of five, seven or eight percent.
This regimen engendered a lot of enthusiasm and became a de facto standard for many physicians based on a single, small Phase II trial. We sought to confirm that the strategy was effective and conducted a randomized Phase III study for patients with Stage IIIA and Stage IIIB disease (Hanna 2007).
A total of 243 patients entered our trial. All patients received cisplatin/etoposide and concurrent radiation at 59.4 Gray. Then, after a rest period of four to eight weeks — and as long as they had not progressed and remained eligible — patients were randomly assigned to either three cycles of docetaxel or observation. We reported several provocative findings. No difference was observed in progression-free survival between the two randomization arms, and no difference was observed in overall survival. The p-value was 0.9 and the curves were completely superimposable (Hanna 2007). We determined that no evidence existed that consolidation docetaxel after chemoradiation therapy improves outcomes but does significantly increase risks for patients, including treatment-related death and serious toxicities such as febrile neutropenia, infections and Grade III/IV pneumonitis.
Track 15
![]() DR LOVE: Can you discuss how vandetanib works and what we know
about it in lung cancer?
DR LOVE: Can you discuss how vandetanib works and what we know
about it in lung cancer?
![]() DR HANNA: Vandetanib is an interesting oral agent. It is a dual EGFR and
VEGFR kinase inhibitor. The questions are, is it as good of an EGFR inhibitor
as erlotinib, and is it as good of a VEGF inhibitor as bevacizumab? Simply
because it hits the same general pathways and targets does not mean that it
will be better than administering two drugs.
DR HANNA: Vandetanib is an interesting oral agent. It is a dual EGFR and
VEGFR kinase inhibitor. The questions are, is it as good of an EGFR inhibitor
as erlotinib, and is it as good of a VEGF inhibitor as bevacizumab? Simply
because it hits the same general pathways and targets does not mean that it
will be better than administering two drugs.
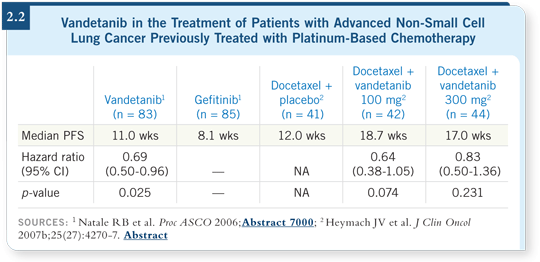
At ASCO 2006, Dr Natale reported the results from a trial comparing vandetanib to gefitinib in the second-line setting. The primary endpoint of the trial was progression-free survival. Vandetanib had a higher response rate and an improved progression-free survival compared to gefitinib (Natale 2006; [2.2]). Also at ASCO 2006, Dr Heymach reported the results of a three-arm, randomized Phase II study in the second-line setting. The patients in the two arms containing vandetanib had what appeared to be an enhanced progression-free survival compared to docetaxel alone (Heymach 2006, 2007b; [2.2]).
A randomized Phase II trial, reported at ASCO 2007, evaluated carboplatin/paclitaxel with or without vandetanib as first-line therapy. The patients receiving vandetanib appeared to have an improved progression-free survival compared to those receiving chemotherapy alone (Heymach 2007a).
Track 18
![]() DR LOVE: How do you approach first-line therapy for patients with
metastatic NSCLC?
DR LOVE: How do you approach first-line therapy for patients with
metastatic NSCLC?
![]() DR HANNA: If a patient has a performance status (PS) of 3 or 4, the right
treatment is best supportive care. If the patient has a PS of 2 and in addition is
experiencing significant loss of appetite, loss of weight and comorbidities, then
I believe the appropriate practice is best supportive care unless he or she is a
never smoker. Then I would consider single-agent erlotinib.
DR HANNA: If a patient has a performance status (PS) of 3 or 4, the right
treatment is best supportive care. If the patient has a PS of 2 and in addition is
experiencing significant loss of appetite, loss of weight and comorbidities, then
I believe the appropriate practice is best supportive care unless he or she is a
never smoker. Then I would consider single-agent erlotinib.
For patients with a PS of 0 or 1 and no contraindications to chemotherapy, I believe a platinum-based, two-drug regimen is standard. For patients who don’t have brain metastases, squamous histology, a history of hemoptysis or uncontrolled hypertension, the addition of bevacizumab is reasonable.
I treat those patients initially with two courses of chemotherapy and repeat their CT scan. If they appear to obtain clinical benefit, I administer four courses of chemotherapy. Because the Sandler study continued patients on bevacizumab (Sandler 2006), I administer it as maintenance until time of progression.
![]() DR LOVE: Which chemotherapy regimens do you think are reasonable to use
in the first-line setting with bevacizumab?
DR LOVE: Which chemotherapy regimens do you think are reasonable to use
in the first-line setting with bevacizumab?
![]() DR HANNA: The only randomized Phase III data are with carboplatin/paclitaxel
(Sandler 2006; [1.1]) and cisplatin/gemcitabine (Manegold 2007; [1.2]).
I believe it’s reasonable to use either of those regimens with bevacizumab.
Bevacizumab is likely to be safe and effective with other regimens, too.
DR HANNA: The only randomized Phase III data are with carboplatin/paclitaxel
(Sandler 2006; [1.1]) and cisplatin/gemcitabine (Manegold 2007; [1.2]).
I believe it’s reasonable to use either of those regimens with bevacizumab.
Bevacizumab is likely to be safe and effective with other regimens, too.
We will be seeing data with docetaxel/platinum and pemetrexed/platinum. At ASCO 2007, Dr Patel reported an improved response rate and acceptable toxicities with carboplatin/pemetrexed and bevacizumab (Patel 2007). I believe those types of regimens would be perfectly acceptable.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Paul A Bunn Jr, MD
- Select publications
Nasser H Hanna, MD
- Select publications
Antoinette J Wozniak, MD
- Select publications
A CME Audio Series and Activity