
 |
|||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you review what we know about the use of bevacizumab
to treat metastatic NSCLC?
DR LOVE: Can you review what we know about the use of bevacizumab
to treat metastatic NSCLC?
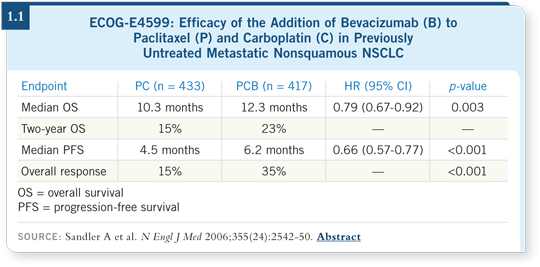
![]() DR BUNN: Two randomized Phase III trials have evaluated bevacizumab
(Sandler 2006; Manegold 2007). One from the US, ECOG-E4599, evaluated
it in combination with carboplatin/paclitaxel. The addition of bevacizumab
demonstrated a survival benefit with a hazard ratio that was clinically relevant (Sandler 2006; [1.1]). So for patients like the ones who were included in
ECOG-E4599 (ie, nonsquamous tumors, no brain metastases, no hemoptysis,
no anticoagulants), I believe most oncologists in the US would use bevacizumab.
I would.
DR BUNN: Two randomized Phase III trials have evaluated bevacizumab
(Sandler 2006; Manegold 2007). One from the US, ECOG-E4599, evaluated
it in combination with carboplatin/paclitaxel. The addition of bevacizumab
demonstrated a survival benefit with a hazard ratio that was clinically relevant (Sandler 2006; [1.1]). So for patients like the ones who were included in
ECOG-E4599 (ie, nonsquamous tumors, no brain metastases, no hemoptysis,
no anticoagulants), I believe most oncologists in the US would use bevacizumab.
I would.
It is reasonable to use bevacizumab for patients who are similar to those who were eligible for ECOG-E4599. However, some questions remain. Bevacizumab has been used for patients with gliomas, and an ongoing study is testing it in patients with brain metastases.
Currently, if you have a patient with a radiated brain metastasis, you should not administer bevacizumab. If a patient is receiving warfarin or subcutaneous heparin, the administration of bevacizumab is also questionable.
Two major differences arose between the AVAiL trial and the ECOG-E4599 trial. The first difference was that the chemotherapy in the AVAiL study was gemcitabine based instead of paclitaxel based. The second difference was that the AVAiL trial used two every three-week doses of bevacizumab, 7.5 mg/kg and 15 mg/kg.
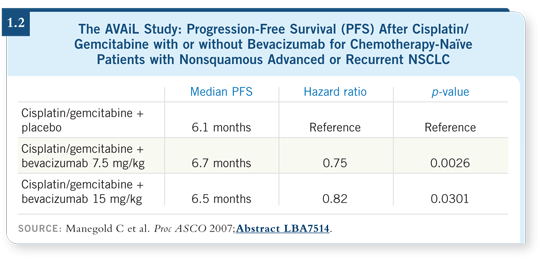
The primary endpoint of the AVAiL study was progression-free survival, and that endpoint was met. That has been presented, but the survival data have not been presented (Manegold 2007; [1.2]).
For progression-free survival, no difference was observed between the 7.5-mg/kg dose and the 15-mg/kg dose every three weeks, and the improvements seemed less striking than those in ECOG-E4599.
We do not know whether the choice of chemotherapy matters or whether the dose of bevacizumab matters. My inclination is to continue using paclitaxel/carboplatin with bevacizumab at a dose of 15 mg/kg every three weeks.
Tracks 5-6
![]() DR LOVE: What do we know about combining other agents with
bevacizumab?
DR LOVE: What do we know about combining other agents with
bevacizumab?
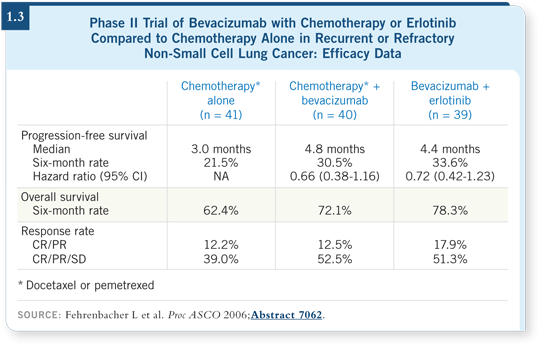
![]() DR BUNN: We have some data in the second-line setting. A randomized Phase
II trial compared docetaxel or pemetrexed alone, docetaxel or pemetrexed with
bevacizumab or erlotinib with bevacizumab.
DR BUNN: We have some data in the second-line setting. A randomized Phase
II trial compared docetaxel or pemetrexed alone, docetaxel or pemetrexed with
bevacizumab or erlotinib with bevacizumab.
The patients in the arms that received bevacizumab seemed to do better than the patients who received chemotherapy alone (Fehrenbacher 2006; [1.3]). The data from this study suggest that two more drugs — pemetrexed and docetaxel — are useful with bevacizumab.
![]() DR LOVE: How do you think through treatment decisions in the second line?
DR LOVE: How do you think through treatment decisions in the second line?
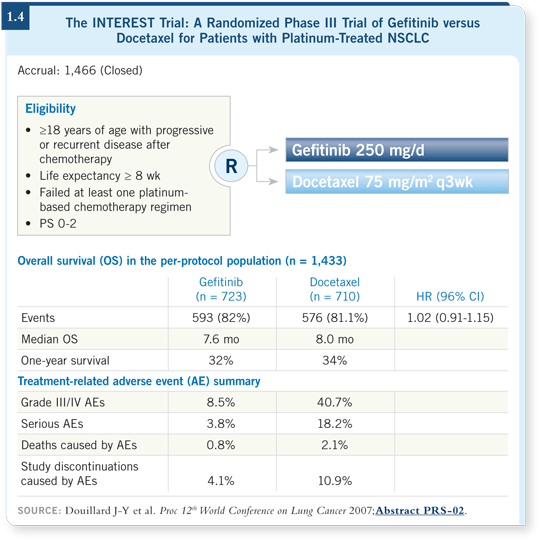
![]() DR BUNN: Second-line treatment is an issue because several drugs —
docetaxel, pemetrexed, gefitinib and erlotinib — have been tested and have a beneficial effect. The largest comparative study, which was presented in Seoul
in 2007, was the INTEREST trial.
DR BUNN: Second-line treatment is an issue because several drugs —
docetaxel, pemetrexed, gefitinib and erlotinib — have been tested and have a beneficial effect. The largest comparative study, which was presented in Seoul
in 2007, was the INTEREST trial.
Docetaxel was compared to gefitinib, and there was absolutely no difference. Those results might lead people to use gefitinib because less toxicity occurred with gefitinib (Douillard 2007; [1.4]).
In the trial comparing pemetrexed to docetaxel, survival was again identical, but pemetrexed was the winner because it was associated with much less toxicity (Hanna 2004). A comparison of pemetrexed versus gefitinib or erlotinib has not been completed.
Track 7
![]() DR LOVE: You mentioned that you tend to use carboplatin/paclitaxel.
What are your thoughts about nab paclitaxel in lung cancer?
DR LOVE: You mentioned that you tend to use carboplatin/paclitaxel.
What are your thoughts about nab paclitaxel in lung cancer?
![]() DR BUNN: The Phase II trials are complete (Reynolds 2007; Hawkins 2007;
Greco 2006), and nab paclitaxel is active. It is similar to paclitaxel but is more convenient. Ongoing randomized Phase III trials are largely being conducted
outside of the US.
DR BUNN: The Phase II trials are complete (Reynolds 2007; Hawkins 2007;
Greco 2006), and nab paclitaxel is active. It is similar to paclitaxel but is more convenient. Ongoing randomized Phase III trials are largely being conducted
outside of the US.
If the findings are negative, that will probably be the end of the drug in lung cancer. If they’re positive, the question is, how would nab paclitaxel be used? And that depends on the magnitude of the differences shown in the Phase III trials. In the US, I believe nab paclitaxel will be used by some clinicians.
Track 13
![]() DR LOVE: Where are we in terms of adjuvant trials for NSCLC?
DR LOVE: Where are we in terms of adjuvant trials for NSCLC?
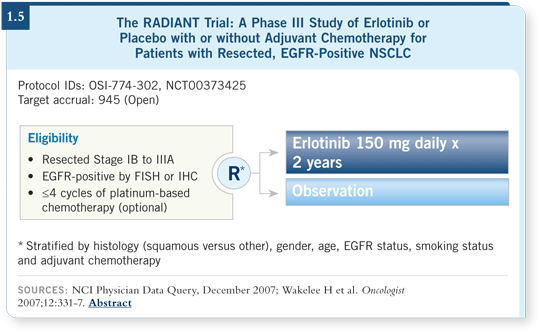
![]() DR BUNN: Currently, the hope is that three important adjuvant trials
— ECOG-E1505, EORTC-08021 and RADIANT — will all accrue. The
RADIANT trial, which is testing erlotinib (1.5), started first. In that study,
patients may or may not have received adjuvant chemotherapy.
DR BUNN: Currently, the hope is that three important adjuvant trials
— ECOG-E1505, EORTC-08021 and RADIANT — will all accrue. The
RADIANT trial, which is testing erlotinib (1.5), started first. In that study,
patients may or may not have received adjuvant chemotherapy.
A patient with Stage IB disease who is not treated in the adjuvant setting is eligible for the study. A patient with Stage II disease who has experienced side effects from surgery and does not want chemotherapy is also eligible, as is the patient who has completed adjuvant chemotherapy. To participate in the study, the patient’s tumor must be IHC-positive and/or FISH-positive for EGFR.
![]() DR LOVE: What about the off-protocol use of erlotinib for such patients as an
alternative strategy?
DR LOVE: What about the off-protocol use of erlotinib for such patients as an
alternative strategy?
![]() DR BUNN: Because we have no data, it is not done much. I have treated one
or two patients that way. It is not something that I recommended, but because
the patients were interested and wanted to do it, I did not prevent them from
doing it. If it were me and my tumor was FISH-positive for EGFR, I would
do it.
DR BUNN: Because we have no data, it is not done much. I have treated one
or two patients that way. It is not something that I recommended, but because
the patients were interested and wanted to do it, I did not prevent them from
doing it. If it were me and my tumor was FISH-positive for EGFR, I would
do it.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Paul A Bunn Jr, MD
- Select publications
Nasser H Hanna, MD
- Select publications
Antoinette J Wozniak, MD
- Select publications
A CME Audio Series and Activity