
| Tracks 1-19 |
| Track 1 |
Introduction |
| Track 2 |
Embryologic theory of cancer of unknown primary |
| Track 3 |
Incidence of cancer of unknown primary |
| Track 4 |
Use of tumor markers in the identification of cancer of unknown primary |
| Track 5 |
Prognosis of cancer of unknown primary |
| Track 6 |
Weekly nanoparticle albumin-bound
(nab) paclitaxel and
carboplatin as first-line therapy
for recurrent or advanced non-small
cell lung cancer (NSCLC) |
| Track 7 |
Shorter infusion time and lack of premedication with nab paclitaxel |
| Track 8 |
Dose and schedule of nab paclitaxel |
| Track 9 |
Tolerability of and sensory neuropathy with nab paclitaxel |
|
| Track 10 |
Considerations in the use of bevacizumab with first-line chemotherapy |
| Track 11 |
Targeting patients for inclusion in adjuvant clinical trials with erlotinib |
| Track 12 |
Use of erlotinib with or without chemotherapy in never smokers |
| Track 13 |
Clinical trial results of adjuvant chemotherapy in Stage IB NSCLC |
| Track 14 |
Selection of adjuvant chemotherapy |
| Track 15 |
Barriers to clinical trial participation |
| Track 16 |
Treatment of Stage IIIB NSCLC |
| Track 17 |
Future directions in the treatment of Stage III disease |
| Track 18 |
Pemetrexed/gemcitabine with bevacizumab in elderly patients with poor performance status |
| Track 19 |
Involvement of community oncologists in clinical research |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: Can you describe the pathogenesis of cancer of unknown
primary?
 DR GRECO: I don’t believe there’s one explanation for all patients with
cancer of unknown primary, but the most attractive theory is what I call an embryologic theory. Basically, cells are misplaced during embryologic migration. The cells in our body that make up all the tissues migrated from a
small blastosphere and grew and differentiated into our organs, and we know
that cells may be misplaced during this process. In other words, pancreas
cells could end up in a lymph node when most of their brethren formed the
pancreas.
DR GRECO: I don’t believe there’s one explanation for all patients with
cancer of unknown primary, but the most attractive theory is what I call an embryologic theory. Basically, cells are misplaced during embryologic migration. The cells in our body that make up all the tissues migrated from a
small blastosphere and grew and differentiated into our organs, and we know
that cells may be misplaced during this process. In other words, pancreas
cells could end up in a lymph node when most of their brethren formed the
pancreas.
 DR LOVE: Can you describe a clinical scenario to illustrate this theory?
DR LOVE: Can you describe a clinical scenario to illustrate this theory?
 DR GRECO: Consider a man who has an enlarged lymph node in his left neck
and no other abnormality anywhere else. A biopsy indicates an adenocarcinoma,
but he’s otherwise healthy. After a full workup, including endoscopy,
CT scanning and PET scanning, everything’s normal.
DR GRECO: Consider a man who has an enlarged lymph node in his left neck
and no other abnormality anywhere else. A biopsy indicates an adenocarcinoma,
but he’s otherwise healthy. After a full workup, including endoscopy,
CT scanning and PET scanning, everything’s normal.
He may have an occult small primary in another site that has spread into the
bloodstream and migrated into a lymph node in his neck, which is the traditional
thinking. The embryologic theory is that the misplaced cell was in his
neck from the time he was born.
Track 3
 DR LOVE:
DR LOVE: Roughly how many cases of cancer of unknown primary
appear in a year in the United States?
 DR GRECO: Approximately 50,000. It’s not rare, and let me clarify that.
Probably a third to a half of the cases are identified as something else. For
example, a patient has liver metastases and an elevated CA19-9 — a marker
associated with pancreatic cancer — but the pancreas is absolutely normal.
More than half of the time, that person’s death certificate will say the patient
died of pancreatic cancer.
DR GRECO: Approximately 50,000. It’s not rare, and let me clarify that.
Probably a third to a half of the cases are identified as something else. For
example, a patient has liver metastases and an elevated CA19-9 — a marker
associated with pancreatic cancer — but the pancreas is absolutely normal.
More than half of the time, that person’s death certificate will say the patient
died of pancreatic cancer.
About 70 to 80 percent of the cases are adenocarcinoma, and the majority
of the rest are poorly differentiated carcinoma. A minority present with one
tumor site, and they have a better prognosis. This is not one group of patients
but rather a heterogeneous group, and several of these patient subgroups are
highly treatable.
 DR LOVE: If you consider the entire heterogeneous group of patients with
unknown primaries, what’s the long-term prognosis?
DR LOVE: If you consider the entire heterogeneous group of patients with
unknown primaries, what’s the long-term prognosis?
 DR GRECO: It’s poor overall. If you treat patients with adenocarcinoma of
unknown etiology, the one-year survival is as good as, if not a little better
than, advanced lung cancer. The two-year survival is around 20 percent of the
patients, and as you go out to five years it settles into 10 to 15 percent.
DR GRECO: It’s poor overall. If you treat patients with adenocarcinoma of
unknown etiology, the one-year survival is as good as, if not a little better
than, advanced lung cancer. The two-year survival is around 20 percent of the
patients, and as you go out to five years it settles into 10 to 15 percent.
Track 6
 DR LOVE:
DR LOVE: Can you describe the study of
nab paclitaxel that you
conducted in non-small cell lung cancer (Allerton 2006)?
 DR GRECO: The study consisted of a weekly schedule of nab paclitaxel and
was designed for patients with advanced or recurrent non-small cell lung
cancer. The patients had to be in reasonable health overall and have normal
organ function. The dose of nab paclitaxel was 100 mg/m2 per week on days
one, eight and 15, and the dose of carboplatin was calculated for an AUC of
six and administered on day one.
DR GRECO: The study consisted of a weekly schedule of nab paclitaxel and
was designed for patients with advanced or recurrent non-small cell lung
cancer. The patients had to be in reasonable health overall and have normal
organ function. The dose of nab paclitaxel was 100 mg/m2 per week on days
one, eight and 15, and the dose of carboplatin was calculated for an AUC of
six and administered on day one.
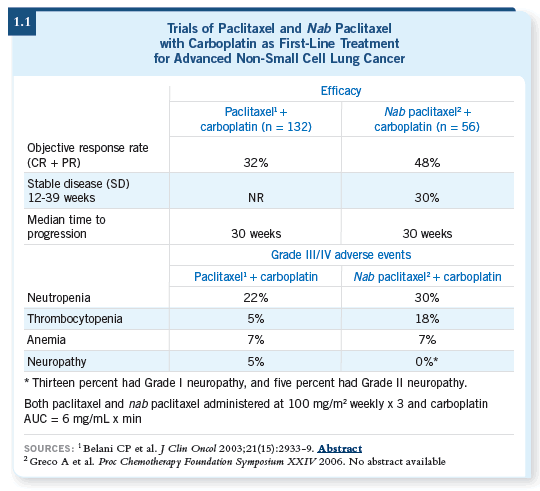
The courses were repeated every 28 days. We treated approximately 60
patients with advanced non-small cell lung cancer, and the response rate
was around 45 percent with confidence intervals that were reasonably tight
(Allerton 2006; [1.1]).
It was a well-tolerated regimen — neutropenia was the most common side
effect, but it wasn’t prolonged or particularly severe in most patients (1.1). The
most troublesome side effect was sensory neuropathy. Unlike the traditional
paclitaxel formulation (Belani 2003), however, the neuropathy associated
with nab paclitaxel was more transient, which has also been observed in breast
cancer clinical trials.
It often resolved within several weeks — sometimes it markedly decreased
within several days. So this Phase II trial showed activity, safety and a
relatively high response rate.
Track 12
 DR LOVE:
DR LOVE: What’s your treatment algorithm for patients with metastatic
NSCLC who are nonsmokers or oligo-smokers with a 15 pack per year
history?
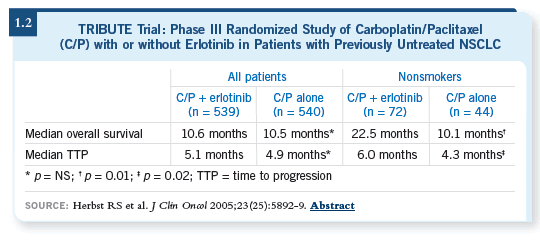
 DR GRECO: Usually erlotinib is on top of the list, either before or combined
with chemotherapy. The data with chemotherapy did not show a benefit, but
those data were from unselected or unenriched populations (Herbst 2005;
[1.2]).
DR GRECO: Usually erlotinib is on top of the list, either before or combined
with chemotherapy. The data with chemotherapy did not show a benefit, but
those data were from unselected or unenriched populations (Herbst 2005;
[1.2]).
We have only anecdotal data for chemotherapy combined with erlotinib
for nonsmokers. My bias is that the combination will be even better than
monotherapy but you will not cure these patients, and erlotinib alone would
be a reasonable first choice for a nonsmoking woman with adenocarcinoma.
 DR LOVE: In the adjuvant setting, would you consider the use of erlotinib for
a nonsmoker?
DR LOVE: In the adjuvant setting, would you consider the use of erlotinib for
a nonsmoker?
 DR GRECO: Yes I would — again, we don’t have good data, but the scientific
logic of it is more important right now. In that situation, I would use erlotinib.
DR GRECO: Yes I would — again, we don’t have good data, but the scientific
logic of it is more important right now. In that situation, I would use erlotinib.
Track 13
 DR LOVE:
DR LOVE: A lot of controversy has emerged since the last ASCO meeting
as a result of the CALGB-9633 data. What’s your take on that?
 DR GRECO: CALGB-9633 was a study conducted to evaluate patients with
Stage IB NSCLC in the postoperative setting. The trial evaluated paclitaxel/carboplatin versus no further treatment, and the study was stopped early after
an interim analysis because the overall and failure-free survival appeared to be
significantly better for the patients receiving adjuvant chemotherapy (Strauss
2006). With additional follow-up, it turned out that the five-year overall
survival was not any different, although progression-free survival still favored
the group receiving adjuvant chemotherapy (Strauss 2006).
DR GRECO: CALGB-9633 was a study conducted to evaluate patients with
Stage IB NSCLC in the postoperative setting. The trial evaluated paclitaxel/carboplatin versus no further treatment, and the study was stopped early after
an interim analysis because the overall and failure-free survival appeared to be
significantly better for the patients receiving adjuvant chemotherapy (Strauss
2006). With additional follow-up, it turned out that the five-year overall
survival was not any different, although progression-free survival still favored
the group receiving adjuvant chemotherapy (Strauss 2006).
The total number of patients on the trial was relatively small, and finding a
certain survival benefit was not statistically likely. In my view, it is hard to
prove this because the power of the study was too low to detect an important
clinical difference. Some would say that is not the reason because other studies
of patients with Stage IB disease showed no benefit, and those studies were
larger — and there’s truth to that.
In the ANITA trial, patients with Stage IB disease did not benefit (Douillard
2005). Some believe the reason patients didn’t benefit was that the ANITA
trial used cisplatin instead of carboplatin.
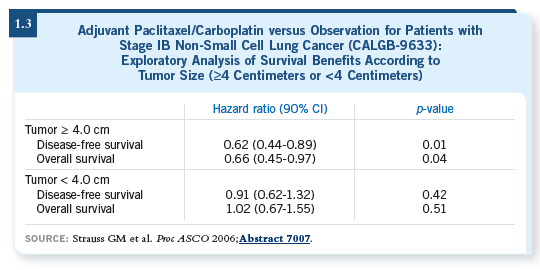
The Canadian trial, led by Dr Francis Shepherd (Winton 2005), showed a
benefit using vinorelbine and cisplatin for patients with Stage IB disease.
It was probably related to the size of the tumors. A retrospective subgroup
analysis of CALGB-9633 (Strauss 2006) suggested that patients with tumors
four centimeters or greater did benefit (1.3).
Track 14
 DR LOVE:
DR LOVE: What kind of data would you want to see in order to use
carboplatin/
nab paclitaxel instead of carboplatin/paclitaxel in the adjuvant
setting?
 DR GRECO: I’d like to see Phase III efficacy data, but toxicity is not a trivial
concern for patients with lung cancer. They’re older, and they tend to have
comorbid conditions. Therefore, I believe that nab paclitaxel could have a role
because the drug is less toxic, particularly in terms of the neuropathy in the
postoperative setting. It combines well with platinum agents, so I believe it
could have a role there.
DR GRECO: I’d like to see Phase III efficacy data, but toxicity is not a trivial
concern for patients with lung cancer. They’re older, and they tend to have
comorbid conditions. Therefore, I believe that nab paclitaxel could have a role
because the drug is less toxic, particularly in terms of the neuropathy in the
postoperative setting. It combines well with platinum agents, so I believe it
could have a role there.
 DR LOVE: Would a Phase III trial in metastatic disease with favorable findings
be enough to adopt nab paclitaxel in the adjuvant setting?
DR LOVE: Would a Phase III trial in metastatic disease with favorable findings
be enough to adopt nab paclitaxel in the adjuvant setting?
 DR GRECO: Yes. Perhaps I jump quicker, and some would say too quickly, but if I had those data in the metastatic setting, I would translate those findings
into the adjuvant setting.
DR GRECO: Yes. Perhaps I jump quicker, and some would say too quickly, but if I had those data in the metastatic setting, I would translate those findings
into the adjuvant setting.
 DR LOVE: So what specifically are you doing first line off protocol in the
adjuvant setting right now?
DR LOVE: So what specifically are you doing first line off protocol in the
adjuvant setting right now?
 DR GRECO: I usually use carboplatin/paclitaxel. The myth is still out there
that cisplatin and carboplatin are really different drugs. In my opinion, they’re
not different except in the rare instance of germ-cell tumors. I’m a proponent
for therapy that doesn’t debilitate patients in the adjuvant setting after thoracic
surgery, and carboplatin is better tolerated in this setting than cisplatin. I know
I am going to get major arguments from some of the purists who’ve conducted
the studies, but that’s okay — I can deal with it.
DR GRECO: I usually use carboplatin/paclitaxel. The myth is still out there
that cisplatin and carboplatin are really different drugs. In my opinion, they’re
not different except in the rare instance of germ-cell tumors. I’m a proponent
for therapy that doesn’t debilitate patients in the adjuvant setting after thoracic
surgery, and carboplatin is better tolerated in this setting than cisplatin. I know
I am going to get major arguments from some of the purists who’ve conducted
the studies, but that’s okay — I can deal with it.
Select publications

