
| Tracks 1-16 |
| Track 1 |
Introduction |
| Track 2 |
Controversies in the adjuvant treatment of Stage IB disease |
| Track 3 |
Incorporating bevacizumab into adjuvant clinical trials |
| Track 4 |
Use of bevacizumab in clinical practice |
| Track 5 |
Use of adjuvant erlotinib in select patients |
| Track 6 |
Study of bevacizumab with either chemotherapy or erlotinib compared to chemotherapy alone for recurrent or refractory NSCLC |
| Track 7 |
Treatment of Stage III NSCLC |
| Track 8 |
New research strategies in Stage III NSCLC |
| Track 9 |
Bevacizumab with chemoradiation therapy for Stage III NSCLC |
|
| Track 10 |
Evaluating biologic agents in the metastatic setting |
| Track 11 |
Treatment algorithm for metastatic NSCLC |
| Track 12 |
Potential advantages of nab paclitaxel |
| Track 13 |
Clinical trial strategies with select patients, targeted agents and tissue correlative studies |
| Track 14 |
Future directions for clinical research in small cell lung cancer |
| Track 15 |
IELCAP study of spiral CT screening for patients at high risk for lung cancer |
| Track 16 |
Biomarker discovery and individualized therapy in lung cancer |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: Can you summarize the current available data on adjuvant
therapy for non-small cell lung cancer?
 DR BELANI: I believe adjuvant therapy has become the standard for patients
with resected non-small cell lung cancer. After 2005, it was the standard for
patients with Stage IB to IIIA disease. Now we have a brewing controversy
regarding whether we should administer adjuvant therapy to patients with
Stage IB disease.
DR BELANI: I believe adjuvant therapy has become the standard for patients
with resected non-small cell lung cancer. After 2005, it was the standard for
patients with Stage IB to IIIA disease. Now we have a brewing controversy
regarding whether we should administer adjuvant therapy to patients with
Stage IB disease.
One issue in the controversy is whether or not it was carboplatin that caused the failure of the carboplatin and paclitaxel regimen for patients with Stage
IB disease in CALGB-9633 (Strauss 2006). At long-term follow-up, the data
failed to show an improvement in overall survival because the hazard ratio fell
from 0.62 to 0.80 and the p-value was not significant. As a word of caution,
that was a small trial, and it is still not completed.
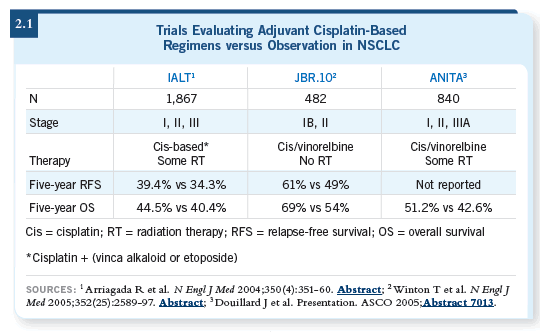
In general, considering the results of the other clinical trials, the JBR.10
study (Winton 2005), the IALT study (Arriagada 2004) and the ANITA trial
(Douillard 2006), adjuvant chemotherapy did play a role in Stage IB disease
because in those trials the chemotherapy was cisplatin based (2.1).
The CALGB-9633 trial has shown in a subset analysis that among patients
who have tumors greater than four centimeters, a benefit still exists (Strauss
2006; [1.3]). But again, we may be reading too much into these subset
analyses, which were not clear endpoints of these clinical studies.
In the clinical setting, for Stage IB disease, I offer chemotherapy to patients,
informing them that in a small subset it has shown a benefit and in another
subset it has not shown a benefit. I let the patient decide whether he or she
wants to receive adjuvant chemotherapy. If the tumor is greater than four
centimeters in size, then I usually suggest that the patient receive it.
 DR LOVE: What about the older patient who has some comorbid conditions
but is still healthy enough to consider therapy?
DR LOVE: What about the older patient who has some comorbid conditions
but is still healthy enough to consider therapy?
 DR BELANI: The older patient should be considered for adjuvant chemotherapy
because the JBR.10 trial reported on the elderly subset of patients.
About 155 patients in the JBR.10 trial were elderly (Winton 2005; Pepe
2006), and a significant benefit was still evident in that population. The
numbers are actually higher than they were in the total patient population: A
20 percent benefit appeared with adjuvant chemotherapy in the elderly population,
and a 15 percent benefit appeared in the overall population.
DR BELANI: The older patient should be considered for adjuvant chemotherapy
because the JBR.10 trial reported on the elderly subset of patients.
About 155 patients in the JBR.10 trial were elderly (Winton 2005; Pepe
2006), and a significant benefit was still evident in that population. The
numbers are actually higher than they were in the total patient population: A
20 percent benefit appeared with adjuvant chemotherapy in the elderly population,
and a 15 percent benefit appeared in the overall population.
Five-year overall survival was 69 percent in the overall population versus 66
percent in the elderly population. So if the performance status was good and
a patient didn’t have any significant problems, I would offer cisplatin-based
chemotherapy to an older patient.
Tracks 3-4
 DR LOVE:
DR LOVE: Where are we in terms of the next generation of adjuvant
trials, particularly with regard to the issue of evaluating bevacizumab?
 DR BELANI: The next adjuvant bevacizumab trial will be ECOG-E1505
(2.2), and it includes three regimens — cisplatin/vinorelbine, cisplatin/docetaxel and cisplatin/gemcitabine — all with or without bevacizumab.
Eligible patients will include those with Stage IB disease who have tumors that are greater than four centimeters in size and those with Stage II or Stage IIIA
disease without mediastinal nodes.
DR BELANI: The next adjuvant bevacizumab trial will be ECOG-E1505
(2.2), and it includes three regimens — cisplatin/vinorelbine, cisplatin/docetaxel and cisplatin/gemcitabine — all with or without bevacizumab.
Eligible patients will include those with Stage IB disease who have tumors that are greater than four centimeters in size and those with Stage II or Stage IIIA
disease without mediastinal nodes.

 DR LOVE: What were your thoughts about the ECOG-E4599 study (Sandler
2005) evaluating carboplatin/paclitaxel with or without bevacizumab in
patients with metastatic disease?
DR LOVE: What were your thoughts about the ECOG-E4599 study (Sandler
2005) evaluating carboplatin/paclitaxel with or without bevacizumab in
patients with metastatic disease?
 DR BELANI: That’s an excellent trial that has shown a significant but modest
benefit for bevacizumab in combination with carboplatin and paclitaxel. A
two-month survival difference appeared, and this is the only trial that has
shown a one-year survival greater than 50 percent in a select group of patients
with metastatic NSCLC.
DR BELANI: That’s an excellent trial that has shown a significant but modest
benefit for bevacizumab in combination with carboplatin and paclitaxel. A
two-month survival difference appeared, and this is the only trial that has
shown a one-year survival greater than 50 percent in a select group of patients
with metastatic NSCLC.
 DR LOVE: How have those data affected your practice?
DR LOVE: How have those data affected your practice?
 DR BELANI: We are using bevacizumab for patients with metastatic nonsquamous
cell carcinoma outside of a study. We are still following the ECOG-E4599
criteria. Outside of a study context, I would not recommend its use
for patients with squamous cell carcinoma, patients with brain metastases or
patients taking anticoagulants.
DR BELANI: We are using bevacizumab for patients with metastatic nonsquamous
cell carcinoma outside of a study. We are still following the ECOG-E4599
criteria. Outside of a study context, I would not recommend its use
for patients with squamous cell carcinoma, patients with brain metastases or
patients taking anticoagulants.
Track 6
 DR LOVE:
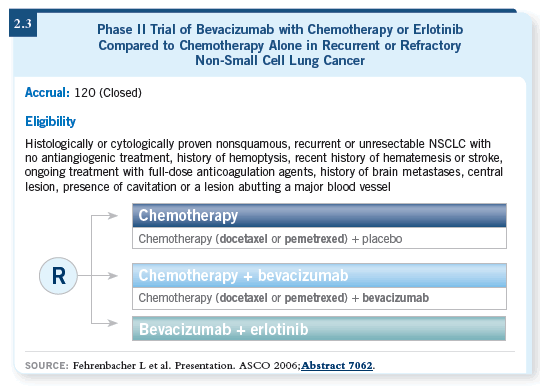
DR LOVE: What are your thoughts about the combination of erlotinib
and bevacizumab and the data that were presented at ASCO (2.3)?
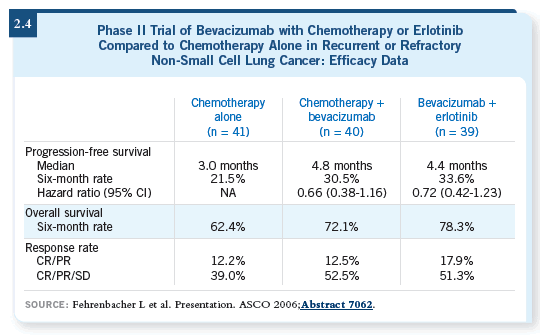
 DR BELANI: I consider it a combination worth pursuing further. A Phase
III trial is definitely warranted in the second-line setting. A second-line trial (Fehrenbacher 2006) compared chemotherapy to chemotherapy with bevacizumab
to erlotinib with bevacizumab. The trial showed that the progression-free
survival is superior in the chemotherapy with bevacizumab arm and in
the erlotinib with bevacizumab arm by about 50 percent compared to chemotherapy
alone (2.4).
DR BELANI: I consider it a combination worth pursuing further. A Phase
III trial is definitely warranted in the second-line setting. A second-line trial (Fehrenbacher 2006) compared chemotherapy to chemotherapy with bevacizumab
to erlotinib with bevacizumab. The trial showed that the progression-free
survival is superior in the chemotherapy with bevacizumab arm and in
the erlotinib with bevacizumab arm by about 50 percent compared to chemotherapy
alone (2.4).
Select publications

