You are here: Home: LCU 1 | 2006 : Thomas J. Lynch, MD

| Tracks 1-21 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Predictors of response to EGFR
TKIs in NSCLC |
| Track 3 |
Development of the Colorado
Index to assist in therapy
selection |
| Track 4 |
Phase II study of gefitinib in
treatment-naïve patients with
Stage IIIB-IV NSCLC and EGFR
mutations |
| Track 5 |
Nonsmoking status as a predictor
of response to EGFR TKIs |
| Track 6 |
Designing and targeting therapies
in NSCLC based on oncogenic
signatures |
| Track 7 |
Research strategies to evaluate
EGFR TKIs in the adjuvant setting |
| Track 8 |
Use of adjuvant TKIs in
nonsmoking patients or those
with the EGFR mutation |
| Track 9 |
Mutations as a means for
discerning mechanisms of
therapeutic resistance |
| Track 10 |
Case discussion: A 51-year-old
nonsmoking woman with
metastatic NSCLC treated with
carboplatin/paclitaxel plus
bevacizumab |
|
| Track 11 |
Risk of hemoptysis in patients
with central or squamous cell
tumors treated with bevacizumab |
| Track 12 |
Potential mechanisms of bevacizumab-associated hemoptysis |
| Track 13 |
ECOG-E4599 and the effect
of bevacizumab on patients
with NSCLC |
| Track 14 |
Use of bevacizumab in
combination with other
chemotherapeutic agents |
| Track 15 |
Clinical investigations of bevacizumab
in the adjuvant setting |
| Track 16 |
Selection of adjuvant
chemotherapy regimens |
| Track 17 |
Selection of patients with Stage
IA disease to receive adjuvant
chemotherapy |
| Track 18 |
Potential utility of neoadjuvant
versus adjuvant chemotherapy |
| Track 19 |
Therapeutic approach to patients
with Stage III NSCLC |
| Track 20 |
SWOG-S9504: Consolidation
docetaxel after chemoradiotherapy
in Stage IIIB NSCLC |
| Track 21 |
Future clinical trial challenges in
advancing adjuvant therapy in
NSCLC |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Would you provide an overview of the predictors of response
to EGFR tyrosine kinase inhibitors? DR LOVE: Would you provide an overview of the predictors of response
to EGFR tyrosine kinase inhibitors? |
 DR LYNCH: The field has progressed rapidly in the past 10 to 12 months in
terms of what we know about predicting response to EGFR TKIs. We’ve seen
a remarkable number of studies, which have been very consistent in demonstrating
a particularly dramatic response to erlotinib and gefitinib in patients
with mutations in the tyrosine kinase domain of the EGFR gene. DR LYNCH: The field has progressed rapidly in the past 10 to 12 months in
terms of what we know about predicting response to EGFR TKIs. We’ve seen
a remarkable number of studies, which have been very consistent in demonstrating
a particularly dramatic response to erlotinib and gefitinib in patients
with mutations in the tyrosine kinase domain of the EGFR gene.
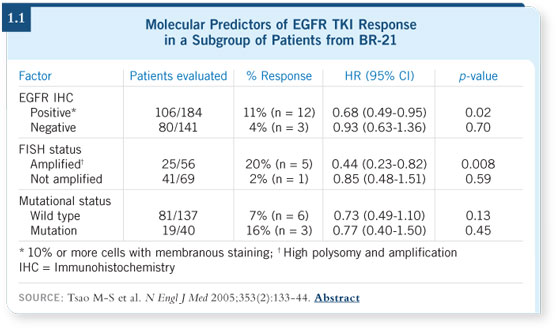
However, when looking at a retrospective subset (Tsao 2005) from the large
randomized trial BR-21 from Canada (Shepherd 2005), we are not able to
associate patient survival with mutations (1.1). Rather, what appeared to be
more important in the BR-21 subset is gene copy number, as measured by
FISH, and immunohistochemistry (IHC) of the EGFR protein.
These are very important measures and may reflect tumors that have a certain
degree of dependence on the epidermal growth factors.
 DR LOVE: Is this information of practical use to an oncologist currently? DR LOVE: Is this information of practical use to an oncologist currently?
 DR LYNCH: Right now, it’s a little unclear. When I see a patient with an
adenocarcinoma — basically, all patients with nonsquamous cell tumors
— I frequently obtain EGFR sequencing up front. If the disease is mutation
positive, the patient qualifies for a clinical trial looking at EGFR TKI therapy
in the first line. DR LYNCH: Right now, it’s a little unclear. When I see a patient with an
adenocarcinoma — basically, all patients with nonsquamous cell tumors
— I frequently obtain EGFR sequencing up front. If the disease is mutation
positive, the patient qualifies for a clinical trial looking at EGFR TKI therapy
in the first line.
 Track 8 Track 8
 DR LOVE: Right now, when you’re evaluating a patient for adjuvant
therapy, do you offer adjuvant erlotinib off trial to a younger patient in his
or her fifties who is either a nonsmoker or has the mutation? DR LOVE: Right now, when you’re evaluating a patient for adjuvant
therapy, do you offer adjuvant erlotinib off trial to a younger patient in his
or her fifties who is either a nonsmoker or has the mutation? |
 DR LYNCH: I don’t, but that’s one of the questions we frequently debate. The
reason I don’t offer a TKI off protocol is based on the results of the SWOG
S0023 study (Kelly 2005), which randomly assigned patients to gefitinib
or placebo after chemoradiation for Stage III lung cancer (1.2). That study
showed patients who received adjuvant gefitinib had a worse outcome, not
statistically significant but very close to being statistically significant, than
patients who received placebo. DR LYNCH: I don’t, but that’s one of the questions we frequently debate. The
reason I don’t offer a TKI off protocol is based on the results of the SWOG
S0023 study (Kelly 2005), which randomly assigned patients to gefitinib
or placebo after chemoradiation for Stage III lung cancer (1.2). That study
showed patients who received adjuvant gefitinib had a worse outcome, not
statistically significant but very close to being statistically significant, than
patients who received placebo.
 DR LOVE: I remember asking you previously what you would do if you were
in that situation — a nonsmoker with a mutation in the adjuvant setting. As I
recall, you said you’d probably opt for treatment. DR LOVE: I remember asking you previously what you would do if you were
in that situation — a nonsmoker with a mutation in the adjuvant setting. As I
recall, you said you’d probably opt for treatment.
 DR LYNCH: I said that I would probably choose treatment because I’d be
willing to accept that risk for myself. So it is something we need to discuss
with patients, to see if they’re willing to accept the risk — which may be an
increased risk of death in this setting. DR LYNCH: I said that I would probably choose treatment because I’d be
willing to accept that risk for myself. So it is something we need to discuss
with patients, to see if they’re willing to accept the risk — which may be an
increased risk of death in this setting.
To be quite frank, we’re not going to have this answer for seven to 10 years.

 Track 15 Track 15
 DR LOVE: Where are we right now in terms of trials evaluating
bevacizumab in the adjuvant setting? Can you speculate whether bevacizumab
might be more or less effective in the adjuvant compared to the
metastatic setting? DR LOVE: Where are we right now in terms of trials evaluating
bevacizumab in the adjuvant setting? Can you speculate whether bevacizumab
might be more or less effective in the adjuvant compared to the
metastatic setting? |
 DR LYNCH: We have a trial just starting at Massachusetts General Hospital
and at the Dana-Farber Cancer Institute in which we’re treating patients with
chemotherapy and bevacizumab in the adjuvant setting. DR LYNCH: We have a trial just starting at Massachusetts General Hospital
and at the Dana-Farber Cancer Institute in which we’re treating patients with
chemotherapy and bevacizumab in the adjuvant setting.
It’s a pilot study and the design is straightforward: 50 people, Phase II, and just
getting off the ground now. We’re looking at the use of bevacizumab/carboplatin/
paclitaxel in the adjuvant setting. Patients must be treated within eight
weeks of surgery, they can’t show any evidence of hemoptysis, and they must
have T2 tumors or greater. Patients with Stage IA disease are not eligible.
 DR LOVE: Do you include patients with squamous cell disease in the trial? DR LOVE: Do you include patients with squamous cell disease in the trial?
 DR LYNCH: In completely resected lung cancer, no squamous cells should
remain so there is no theoretical reason that patients should bleed. I don’t
think there’s any reason to believe bevacizumab would not be beneficial in
that group of patients, so we will be including patients with squamous cell
disease in the trial. DR LYNCH: In completely resected lung cancer, no squamous cells should
remain so there is no theoretical reason that patients should bleed. I don’t
think there’s any reason to believe bevacizumab would not be beneficial in
that group of patients, so we will be including patients with squamous cell
disease in the trial.
 Track 20 Track 20
 DR LOVE: Do you use chemotherapy after chemoradiotherapy in patients
with Stage IIIB disease? The SWOG regimen utilizing docetaxel maintenance
has generated a lot of excitement. Can you talk a little bit about
that and discuss the updated data (Gandara 2005) that were presented at
ASCO? DR LOVE: Do you use chemotherapy after chemoradiotherapy in patients
with Stage IIIB disease? The SWOG regimen utilizing docetaxel maintenance
has generated a lot of excitement. Can you talk a little bit about
that and discuss the updated data (Gandara 2005) that were presented at
ASCO? |
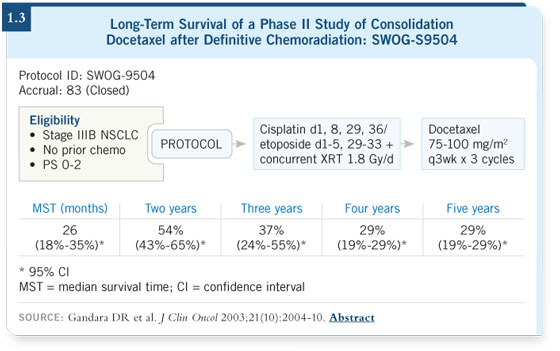
 DR LYNCH: The SWOG-S9504 data look very encouraging. Etoposide/
platinum was used in a 50-50 combination in which you administer 50 mg/m2 of platinum on day one and day eight and 50 mg/m2 of etoposide on days one
to five. That way, full doses of etoposide/platinum are received in cycle one
and cycle two. When you administer that combination with radiation, you
have 12 days of overlap. I believe that’s a very good regimen. Cisplatin/etoposide
with radiation is followed by three cycles of docetaxel, and I believe this
is a very good approach. The SWOG data showed a median survival of 26
months at 32 months follow-up (1.3). DR LYNCH: The SWOG-S9504 data look very encouraging. Etoposide/
platinum was used in a 50-50 combination in which you administer 50 mg/m2 of platinum on day one and day eight and 50 mg/m2 of etoposide on days one
to five. That way, full doses of etoposide/platinum are received in cycle one
and cycle two. When you administer that combination with radiation, you
have 12 days of overlap. I believe that’s a very good regimen. Cisplatin/etoposide
with radiation is followed by three cycles of docetaxel, and I believe this
is a very good approach. The SWOG data showed a median survival of 26
months at 32 months follow-up (1.3).
However, I tend to use weekly carboplatin with a taxane for patients with
reasonable functional status who either have impaired organ function or
marginal performance status, or for people who I don’t think can tolerate
cisplatin. Generally, I tend to be a very big believer in the SWOG trial until
convinced otherwise.
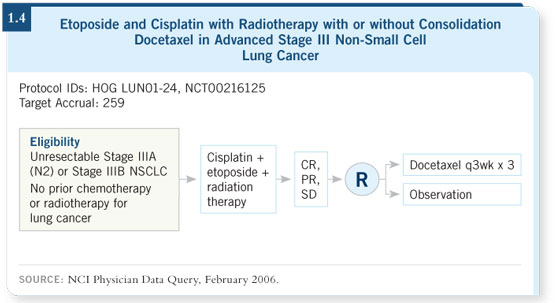
We’ll have some data coming out from a Hoosier Oncology Group study
(LUN01-24; [1.4]) which evaluated etoposide and cisplatin with radiation
therapy followed by docetaxel, asking whether the posterior docetaxel is
important in that setting.
This will be a very important trial. Some subsets of Karen Kelly’s SWOG-
0023 trial do suggest that the cisplatin/etoposide followed by docetaxel arm
appears to be holding up pretty well.
Select publications
|

