You are here: Home: LCU 1 | 2006 : Kathy S Albain, MD

| Tracks 1-13 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
ECOG-E4599: Translating first-line
bevacizumab data into
clinical practice |
| Track 3 |
Selection of first-line therapy
for patients ineligible for
ECOG-E4599 |
| Track 4 |
Incorporating erlotinib into the
management of metastatic
NSCLC |
| Track 5 |
Identifying predictors of response
to EGFR TKIs |
| Track 6 |
Use of adjuvant chemotherapy in
patients with lower-risk disease |
| Track 7 |
Incorporating targeted therapies
into adjuvant clinical trials |
| Track 8 |
Continuation of bevacizumab
after disease progression |
|
| Track 9 |
RTOG-9309: Radiotherapy
concurrent with cisplatin/
etoposide with or without surgical
resection in Stage IIIA NSCLC |
| Track 10 |
RTOG-9309: Higher mortality
associated with pneumonectomy |
| Track 11 |
Docetaxel consolidation after
induction chemoradiation in
Stage IIIB disease |
| Track 12 |
Chemoradiation therapy for
patients with Stage III disease |
| Track 13 |
SWOG-S0229: Pulmonary
rehabilitation education with or
without exercise training |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Would you comment on ECOG-E4599, which evaluated
bevacizumab in combination with chemotherapy in patients with
metastatic disease? DR LOVE: Would you comment on ECOG-E4599, which evaluated
bevacizumab in combination with chemotherapy in patients with
metastatic disease? |
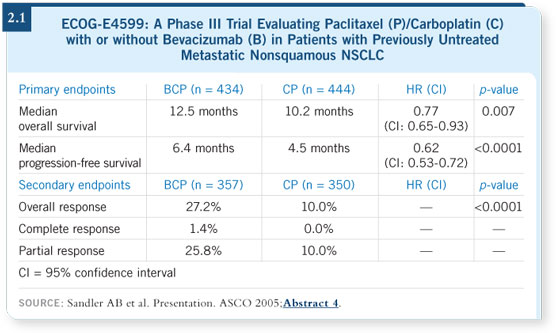
 DR ALBAIN: The addition of bevacizumab makes carboplatin/paclitaxel a
better regimen. If you want to utilize carboplatin and paclitaxel in the front-line
setting and the patient meets the criteria for ECOG-E4599 (nonsquamous
histology and no history of uncontrolled hypertension, bleeding or clotting),
you should probably also administer bevacizumab (Sandler 2005; [2.1]). DR ALBAIN: The addition of bevacizumab makes carboplatin/paclitaxel a
better regimen. If you want to utilize carboplatin and paclitaxel in the front-line
setting and the patient meets the criteria for ECOG-E4599 (nonsquamous
histology and no history of uncontrolled hypertension, bleeding or clotting),
you should probably also administer bevacizumab (Sandler 2005; [2.1]).
 DR LOVE: How do you approach the selection of chemotherapy in the first-line
setting for patients with metastatic NSCLC, and where does bevacizumab fit in? DR LOVE: How do you approach the selection of chemotherapy in the first-line
setting for patients with metastatic NSCLC, and where does bevacizumab fit in?
 DR ALBAIN: Our goal is to enroll our patients on clinical trials. In the
Southwest Oncology Group, we’ve just finished a study (SWOG-S0342)
with cetuximab, either concurrent with or sequenced after carboplatin
and paclitaxel. DR ALBAIN: Our goal is to enroll our patients on clinical trials. In the
Southwest Oncology Group, we’ve just finished a study (SWOG-S0342)
with cetuximab, either concurrent with or sequenced after carboplatin
and paclitaxel.
As I mentioned previously, if you want to use carboplatin and paclitaxel as
your main regimen in this patient population, then you should add bevacizumab
(Sandler 2005).
There is a preclinical rationale for the combination of bevacizumab with a
taxane, and a lot of work is being done to evaluate other potentially additive
and/or synergistic combinations. So, hopefully, there’ll be more agents
to work with. I also use other regimens: cisplatin/docetaxel, cisplatin/
gemcitabine, and a number of others.
 DR LOVE: How do you decide between those options on a case-by-case basis?
What are some of the factors you consider? DR LOVE: How do you decide between those options on a case-by-case basis?
What are some of the factors you consider?
 DR ALBAIN: I consider the patient’s overall performance status and comorbid
illnesses. So if the patient, for example, has diabetes that’s quite difficult to
control, you may stay away from a regimen that requires steroids for a few
days. There are a lot of things that play into the decision-making in advanced
lung cancer. Patients often have a spectrum of other illnesses that go along
with prior smoking — poor lung function, et cetera. DR ALBAIN: I consider the patient’s overall performance status and comorbid
illnesses. So if the patient, for example, has diabetes that’s quite difficult to
control, you may stay away from a regimen that requires steroids for a few
days. There are a lot of things that play into the decision-making in advanced
lung cancer. Patients often have a spectrum of other illnesses that go along
with prior smoking — poor lung function, et cetera.
 Track 7 Track 7
 DR LOVE: Where do you see things heading with the next generation of
adjuvant trials in NSCLC? DR LOVE: Where do you see things heading with the next generation of
adjuvant trials in NSCLC? |
 DR ALBAIN: We do need an adjuvant bevacizumab trial, and one is being
designed right now and will be conducted by the Intergroup. I believe that we also have to get erlotinib back in trials, because I can’t imagine that it
wouldn’t be helpful for minimal residual disease as opposed to very advanced
chemotherapy-refractory disease. DR ALBAIN: We do need an adjuvant bevacizumab trial, and one is being
designed right now and will be conducted by the Intergroup. I believe that we also have to get erlotinib back in trials, because I can’t imagine that it
wouldn’t be helpful for minimal residual disease as opposed to very advanced
chemotherapy-refractory disease.
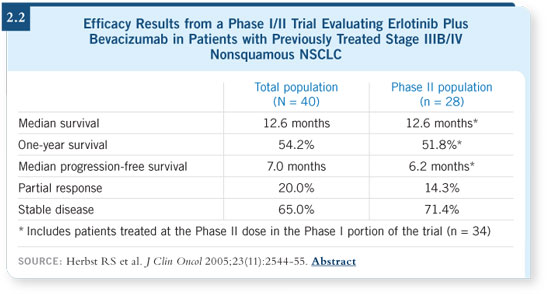
 DR LOVE: What are your thoughts about the report by Herbst and Sandler
evaluating the combination of bevacizumab and erlotinib (Herbst 2005; [2.2])? DR LOVE: What are your thoughts about the report by Herbst and Sandler
evaluating the combination of bevacizumab and erlotinib (Herbst 2005; [2.2])?
 DR ALBAIN: That’s a very interesting trial, and we’re participating in the
follow-up study. It’s an extremely provocative result, and it is worth testing the
combination of agents. Combining targeted agents is going to be important for
those tumors that aren’t driven by a dominant single pathway. DR ALBAIN: That’s a very interesting trial, and we’re participating in the
follow-up study. It’s an extremely provocative result, and it is worth testing the
combination of agents. Combining targeted agents is going to be important for
those tumors that aren’t driven by a dominant single pathway.
 Tracks 9-10 Tracks 9-10
 DR LOVE: Would you talk about the RTOG-9309 study? DR LOVE: Would you talk about the RTOG-9309 study? |
 DR ALBAIN: RTOG-9309 evolved because of the large number of Phase II
trials conducted in the late 1980s and early 1990s that showed the feasibility of
sending patients for a resection following chemoradiotherapy. DR ALBAIN: RTOG-9309 evolved because of the large number of Phase II
trials conducted in the late 1980s and early 1990s that showed the feasibility of
sending patients for a resection following chemoradiotherapy.
Other trials were studying chemotherapy alone, but at least in the Southwest
Oncology Group’s series of pilot studies, we were evaluating a concurrent
chemoradiotherapy program that showed a lot of promise.
That was brought into two studies — the first a trimodality study with
surgery after the chemoradiation and the second with straight chemoradiation.
These trials not only showed feasibility, but they also raised some concerns
about a higher rate of acute respiratory distress syndrome (ARDS) after
surgery.
Additionally, these trials identified a group of patients who — even though
they had high-volume disease prior to therapy — at the time of surgery would
have no remaining disease in their mediastinal nodes. The pilot study observed patients with Stage IIIA (pN2) and IIIB (nonpleural effusion) disease. The big
debate, then, was whether surgery really contributes to survival.
Thus, SWOG started a Phase III trial while several other Phase III trials were
going on. The Intergroup decided to shut all of them down and start a new
one (RTOG-9309), which was identical to the SWOG design but was run by
the RTOG. RTOG-9309 limped along in its accrual for a number of years.
It was an extremely difficult study to discuss with patients because you had to
inform them of the possibility of a higher rate of postoperative death. The trial
finally recorded enough events for reporting, which were fewer than projected
because of the long duration of accrual.
The first report showed a marked improvement in progression-free survival
with the addition of surgery. Reproducing what we had seen in the pilot
study, those patients with mediastinal nodes that were no longer positive after
chemoradiotherapy seemed to have the best outcome.
The overall survival, however, showed no difference; the tail of the curve was
a little bit separated, but the p-value was not significant (Albain 2003).
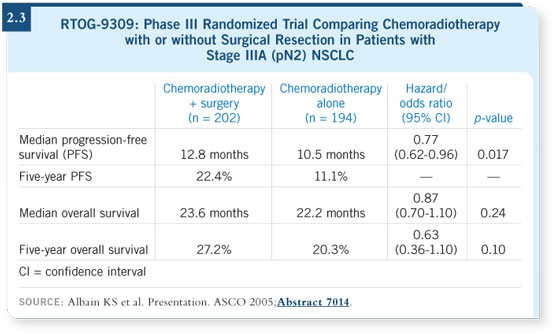
A final survival report was planned when more data were available, and we
presented it at ASCO 2005. We showed the same overall results in terms of
progression-free survival and overall survival (Albain 2005; [2.3]).
We looked very carefully at the postoperative deaths. We found — just as
we had in the pilot trial — that the majority of the postoperative deaths
occurred in patients who had undergone a pneumonectomy. These deaths
were predominantly from ARDS, just as other postoperative deaths we have
reported. The trial was not able to show an overall survival advantage because
of these postoperative deaths (Albain 2005).
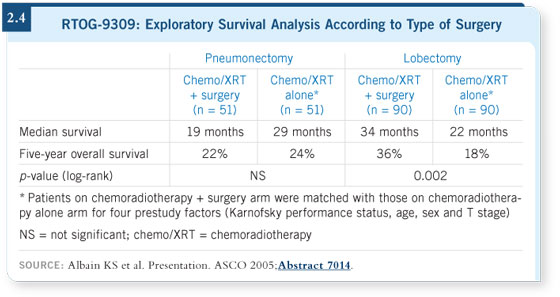
Our statistician performed an exploratory analysis matching the patients who
had undergone pneumonectomies to a similar group in the nonsurgical arm.
The same process was followed with the patients who had undergone lobectomies.
A dramatic split favored the lobectomy group over chemoradiotherapy
alone, whereas the patients who had pneumonectomies had a worse survival
rate than a matched group that did not receive surgery (Albain 2005; [2.4]).
That analysis was exploratory, but it answered the question, “What do I do
in practice?” These were the types of patients for whom the standard of care
would be chemotherapy and radiation. These were not the patients with very
minimal N2 disease, who could still undergo surgery.
If I see a patient with mediastinal disease who fits the criteria for the study, is
suited to undergoing a lobectomy, is fit and has a good performance status and
good pulmonary function, we discuss the study results.
Select publications
|

