
| Tracks 1-16 |
| Track 1 |
Approaches to chemoRT for Stage III NSCLC |
| Track 2 |
HOG LUN 01-24: Cisplatin/ etoposide and concurrent radiation therapy with or without consolidation docetaxel for inoperable Stage III NSCLC |
| Track 3 |
Scientific and clinical interpretation of the SWOG-S0023 trial results |
| Track 4 |
Clinical algorithm for the use
of erlotinib in patients with
metastatic NSCLC |
| Track 5 |
Investigation of adjuvant erlotinib
in target-enriched patient
populations |
| Track 6 |
Clinical use of adjuvant erlotinib
for nonsmokers with an EGFR
gene mutation |
| Track 7 |
Clinical implications of HOG LUN
01-24 |
|
| Track 8 |
AVAiL: Cisplatin/gemcitabine with or without bevacizumab for chemotherapy-naïve advanced or recurrent nonsquamous NSCLC |
| Track 9 |
Dosing of bevacizumab |
| Track 10 |
Tolerability of bevacizumab |
| Track 11 |
Selection of first-line therapy for patients with metastatic NSCLC |
| Track 12 |
Selection of second-line therapy for patients with metastatic NSCLC |
| Track 13 |
Heterogeneity among PS2 patients: Implications for treatment |
| Track 14 |
Use of erlotinib for PS2 patients with no tumor-related symptoms |
| Track 15 |
Cisplatin/docetaxel as adjuvant therapy for NSCLC |
| Track 16 |
Treatment approach for Stage IB
disease |
|
|
Select Excerpts from the Interview
Tracks 4-5
 DR LOVE:
DR LOVE: What do you think about the trials that are evaluating the
adjuvant use of erlotinib in enriched populations (ie, those with EGFR-positive
tumors)?
 DR EDELMAN: Erlotinib is a fascinating agent because it has shown efficacy in
nonsmokers, never smokers and women with adenocarcinomas and bronchoalveolar
carcinoma features. Patients with these characteristics are coming into
my office with increasing frequency. I’m seeing two to three never smokers
a month in my clinic and an increasing number of patients who smoked for only one year or so. For an enriched population in which you believe that the
EGFR marker is present — either because of positive prognostic factors or
because you’ve actually tested for it — adjuvant study of erlotinib is reasonable.
We need to approach the use of these drugs in a more intelligent fashion,
and I believe this is the way to do it.
DR EDELMAN: Erlotinib is a fascinating agent because it has shown efficacy in
nonsmokers, never smokers and women with adenocarcinomas and bronchoalveolar
carcinoma features. Patients with these characteristics are coming into
my office with increasing frequency. I’m seeing two to three never smokers
a month in my clinic and an increasing number of patients who smoked for only one year or so. For an enriched population in which you believe that the
EGFR marker is present — either because of positive prognostic factors or
because you’ve actually tested for it — adjuvant study of erlotinib is reasonable.
We need to approach the use of these drugs in a more intelligent fashion,
and I believe this is the way to do it.

Obviously in a resected population, you can test for the presence of EGFR by
FISH or gene mutations — whatever your favorite method is.
Track 8
 DR LOVE:
DR LOVE: How do you approach the clinical use of bevacizumab in
metastatic NSCLC?
 DR EDELMAN: I’ve held fairly closely to the ECOG-E4599 eligibility criteria
(Sandler 2005; [2.1]). Patients are concerned about the risk of hemoptysis, but
again, viewing this in the aggregate, patients fared better with bevacizumab.
DR EDELMAN: I’ve held fairly closely to the ECOG-E4599 eligibility criteria
(Sandler 2005; [2.1]). Patients are concerned about the risk of hemoptysis, but
again, viewing this in the aggregate, patients fared better with bevacizumab.
They live longer, so if we have patients who would have been eligible for that,
we approach them about the use of bevacizumab.
I have used bevacizumab pretty much as it was used on E4599 with carboplatin/
paclitaxel. The only difference is that I tend to use less cytotoxic
chemotherapy — I use four cycles, not six, and I base that on my belief that
the evidence is pretty compelling that cytotoxics do not aid you after four
courses of therapy. I could certainly be criticized, but I believe it’s a reasonable
approach and it’s well tolerated.
Track 15
 DR LOVE:
DR LOVE: What chemotherapy regimen do you usually utilize as adjuvant
therapy?
 DR EDELMAN: Generally, cisplatin and docetaxel because I believe the weight
of data supports a cisplatin-based regimen (2.2). If one wants to be completely
data driven, cisplatin/vinorelbine is probably the most validated regimen out
there, but it’s difficult to administer. In Stage IV disease, cisplatin/docetaxel
is at least as good, possibly even superior, and probably better tolerated than
cisplatin/vinorelbine, so I consider that a reasonable regimen.
DR EDELMAN: Generally, cisplatin and docetaxel because I believe the weight
of data supports a cisplatin-based regimen (2.2). If one wants to be completely
data driven, cisplatin/vinorelbine is probably the most validated regimen out
there, but it’s difficult to administer. In Stage IV disease, cisplatin/docetaxel
is at least as good, possibly even superior, and probably better tolerated than
cisplatin/vinorelbine, so I consider that a reasonable regimen.
If someone told me that he or she intended to administer cisplatin/vinorelbine,
I would not argue. The combination of cisplatin/gemcitabine is also reasonable.
The crucial component in this combination is the platinum.
Despite all the controversy, I believe carboplatin/paclitaxel is also reasonable.
Another key issue is adjuvant therapy for Stage IB disease. It has been pointed
out that to conduct an adequately powered study of patients with Stage IB
disease, you’d have to enroll about 2,000 patients.
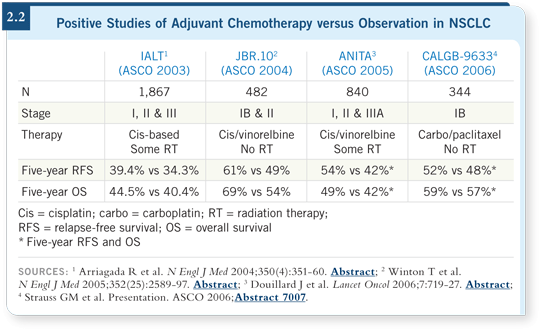
So the CALGB carboplatin/paclitaxel study (Strauss 2006) that showed an
improvement in progression-free survival in Stage IB disease was probably
underpowered.
If you consider the subgroup of patients with tumors of four centimeters or
greater (Strauss 2006), those patients clearly fared better with the chemotherapy.
I don’t believe carboplatin/paclitaxel is inactive in this setting
— occasionally we use that. We use it because some patients cannot tolerate
cisplatin-based therapy.
It is not unusual for us to start with a cisplatin-based therapy and switch the
patient after one or two cycles because he or she cannot tolerate it. So for
their final couple of cycles, these patients are switched to a carboplatin-based
regimen.
Select Publications

