
| Tracks 1-22 |
| Track 1 |
Response to tyrosine kinase
inhibitor (TKI) therapy based on
EGFR mutation status |
| Track 2 |
Mechanism of action of the TKIs |
| Track 3 |
Factors influencing EGFR
mutation status |
| Track 4 |
Techniques for assessing EGFR
mutations |
| Track 5 |
Response to TKIs in nonsmokers
without EGFR mutations |
| Track 6 |
Assessing EGFR mutations using analysis of free DNA in circulating blood |
| Track 7 |
Clinical trials incorporating the assessment of EGFR mutations |
| Track 8 |
Clinical use of EGFR mutation testing |
| Track 9 |
Use of erlotinib in patients with EGFR mutations |
| Track 10 |
Treatment algorithm for the management of metastatic NSCLC |
| Track 11 |
Tolerability of carboplatin/ paclitaxel and bevacizumab |
| Track 12 |
Approach to second-line therapy for metastatic NSCLC |
|
| Track 13 |
AVAiL trial: Cisplatin/gemcitabine with or without bevacizumab in patients with chemotherapy-naïve, advanced or recurrent nonsquamous NSCLC |
| Track 14 |
ECOG-E1505: Adjuvant chemotherapy with or without bevacizumab in completely resected Stage IB to IIIA NSCLC |
| Track 15 |
Tolerability of adjuvant bevacizumab |
| Track 16 |
Adjuvant therapy for Stage IB NSCLC |
| Track 17 |
Nanoparticle albumin-bound (nab) paclitaxel in lung cancer |
| Track 18 |
Data with vandetanib (ZD6474) in lung cancer |
| Track 19 |
Tolerability of vandetanib |
| Track 20 |
Phase II randomized trial of vandetanib and docetaxel |
| Track 21 |
Ongoing trials with vandetanib |
| Track 22 |
Prophylactic cranial radiation
therapy for extensive-stage small
cell lung cancer |
|
|
Select Excerpts from the Interview
Track 1
 DR LOVE:
DR LOVE: Can you provide an overview of the tumor mutations that
are associated with increased response to EGFR TKIs in non-small cell
carcinoma?
 DR JOHNSON: We were involved in the discovery of a correlation between genetic changes in the epidermal growth factor receptor (EGFR) and the likelihood
of response to treatment with either gefitinib or erlotinib (Lynch 2004;
Jackman 2006).
DR JOHNSON: We were involved in the discovery of a correlation between genetic changes in the epidermal growth factor receptor (EGFR) and the likelihood
of response to treatment with either gefitinib or erlotinib (Lynch 2004;
Jackman 2006).
We observed a subgroup of patients who had dramatic, long-lived
responses (1.1), and that was unusual in NSCLC. Subsequently, my colleagues Dr Tom Lynch and Dr Lecia Sequist led a trial
that prospectively identified EGFR mutations in patients with newly diagnosed,
previously untreated NSCLC.
Patients with EGFR mutations were treated with
gefitinib. We reported the results for 31 patients, with a response rate of approximately
60 percent and a time to progression of about one year (Sequist 2007).
Whether those patients with mutations would have done just as well with
chemotherapy remains unknown.
Track 9
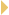 DR LOVE:
DR LOVE: Would you comment on the nonprotocol management of
patients with EGFR mutations and phenotypic predictors of response,
such as nonsmoking status?
 DR JOHNSON: We believe the place to begin to answer that question is in
first-line treatment of metastatic disease for patients with previously untreated
NSCLC. Trials to answer that question — how patients fare with the EGFR
TKIs versus those treated with conventional chemotherapy — will be available
within the next two years.
DR JOHNSON: We believe the place to begin to answer that question is in
first-line treatment of metastatic disease for patients with previously untreated
NSCLC. Trials to answer that question — how patients fare with the EGFR
TKIs versus those treated with conventional chemotherapy — will be available
within the next two years.
That question may be more difficult to answer in the adjuvant setting.
 DR LOVE: What about in the first-line metastatic setting, off protocol?
DR LOVE: What about in the first-line metastatic setting, off protocol?
 DR JOHNSON: The agents aren’t approved for first-line therapy, but we have
had trials with first-line erlotinib and gefitinib for the past five years in our
institution. We’ve considered it when a patient tests mutation-positive and
wouldn’t otherwise qualify for a trial.
DR JOHNSON: The agents aren’t approved for first-line therapy, but we have
had trials with first-line erlotinib and gefitinib for the past five years in our
institution. We’ve considered it when a patient tests mutation-positive and
wouldn’t otherwise qualify for a trial.
Tracks 10-12
 DR LOVE:
DR LOVE: What’s your algorithm for the management of metastatic
disease in the clinical setting for patients who are not in the EGFR-enriched
populations — an average patient, a smoker, et cetera?
 DR JOHNSON: We follow the Eastern Cooperative Oncology Group (ECOG)
algorithm. For patients with adenocarcinoma without brain metastasis, serious
cardiovascular or cerebrovascular problems or clotting, we recommend paclitaxel,
carboplatin and bevacizumab.
DR JOHNSON: We follow the Eastern Cooperative Oncology Group (ECOG)
algorithm. For patients with adenocarcinoma without brain metastasis, serious
cardiovascular or cerebrovascular problems or clotting, we recommend paclitaxel,
carboplatin and bevacizumab.
For patients with SCC, brain metastasis or hemoptysis, we administer paclitaxel
and carboplatin. We try to utilize the same drugs off study as we do on
study. For patients with a number of serious medical issues, we’ll use a single
agent such as vinorelbine.
 DR LOVE: What’s been your experience with the regimen of carboplatin/
paclitaxel with bevacizumab, particularly in terms of the side effects and
toxicity?
DR LOVE: What’s been your experience with the regimen of carboplatin/
paclitaxel with bevacizumab, particularly in terms of the side effects and
toxicity?
 DR JOHNSON: Side effects include hypertension and an increased risk of
clotting, bleeding and proteinuria, which are all manageable. We also see an
increased risk of deep venous thrombosis and pulmonary emboli.
DR JOHNSON: Side effects include hypertension and an increased risk of
clotting, bleeding and proteinuria, which are all manageable. We also see an
increased risk of deep venous thrombosis and pulmonary emboli.
 DR LOVE: How do you approach second-line therapy for patients with NSCLC?
DR LOVE: How do you approach second-line therapy for patients with NSCLC?
 DR JOHNSON: For patients in second-line therapy off study who have been
treated with two agents — most commonly carboplatin/paclitaxel in our
setting — and have a good response and go off therapy for an extended period,
we’ll commonly go back to docetaxel as second-line therapy.
DR JOHNSON: For patients in second-line therapy off study who have been
treated with two agents — most commonly carboplatin/paclitaxel in our
setting — and have a good response and go off therapy for an extended period,
we’ll commonly go back to docetaxel as second-line therapy.
For a patient who shows a mediocre response, we will commonly use erlotinib
as the second agent. We often use pemetrexed as the third-line agent for the
patients who don’t quite fit into classic clinical response categories.
For almost everybody off study, we use one of the three approved agents for
second-line treatment — pemetrexed, docetaxel or erlotinib.
Tracks 18-19
 DR LOVE:
DR LOVE: Which of the new biologic agents show promise and may be
coming into the clinic in the near future?
 DR JOHNSON: One of the classes of agents I believe will likely find a place
is the targeted kinases, such as those that include a VEGF receptor blockade.
Sunitinib and sorafenib have been approved for kidney cancer, and a response rate of 10 to 12 percent has been recorded with sunitinib among previously
treated patients with NSCLC (Socinski 2006).
DR JOHNSON: One of the classes of agents I believe will likely find a place
is the targeted kinases, such as those that include a VEGF receptor blockade.
Sunitinib and sorafenib have been approved for kidney cancer, and a response rate of 10 to 12 percent has been recorded with sunitinib among previously
treated patients with NSCLC (Socinski 2006).
The other agent I work with is vandetanib, which when combined with
docetaxel reached its primary endpoint of prolonging progression-free survival
(Heymach 2006). It is now being tested in a large trial to find out if it
increases response rates.
We have also found that a number of patients can be maintained on vandetanib
from six months up to two years. We believe that represents a particularly
sensitive subset, and we hope to identify what conveys such sensitivity in
those particular tumors.
 DR LOVE: What is the proposed mechanism of action of vandetanib?
DR LOVE: What is the proposed mechanism of action of vandetanib?
 DR JOHNSON: Vandetanib is an inhibitor of the VEGF receptor and the
EGFR. It inhibits the VEGF receptor II at a level about five times lower than
the EGFR.
DR JOHNSON: Vandetanib is an inhibitor of the VEGF receptor and the
EGFR. It inhibits the VEGF receptor II at a level about five times lower than
the EGFR.
When you use a higher dose of vandetanib, 300 milligrams a day,
the patients don’t do as well as when you administer a lower dose, 100 milligrams
a day, within a randomized Phase II trial. Does that have to do with
toxicity, in that if you use a lower dose you can administer it for a longer period of
time, or is it that with the lower dose you block the VEGF II without blocking the
EGFR? That’s currently under investigation.
 DR LOVE: What are the side effects and toxicities of vandetanib?
DR LOVE: What are the side effects and toxicities of vandetanib?
 DR JOHNSON: Rash, diarrhea and, as with many of these agents, prolongation
in the QTc interval — the length of time it takes for the heart to repolarize.
Thus far, no clinical increased risk of arrhythmia has been recorded. Another
effect that can occur is increased sensitivity to the sun. We also see elevation
of blood pressure but less proteinuria than with bevacizumab.
DR JOHNSON: Rash, diarrhea and, as with many of these agents, prolongation
in the QTc interval — the length of time it takes for the heart to repolarize.
Thus far, no clinical increased risk of arrhythmia has been recorded. Another
effect that can occur is increased sensitivity to the sun. We also see elevation
of blood pressure but less proteinuria than with bevacizumab.
In terms of bleeding, randomized Phase II studies have evaluated vandetanib
at two different doses with docetaxel versus docetaxel alone (Heymach 2006),
and an up-front study has been conducted of vandetanib alone versus vandetanib
and paclitaxel/carboplatin or paclitaxel/carboplatin alone (Heymach 2007).
No increased risk of bleeding has been found among those patients, and that
includes the subsets of patients with brain metastasis and SCC excluded from
the trials with bevacizumab.
Track 22
 DR LOVE:
DR LOVE: Can you discuss the ASCO presentation evaluating prophylactic
cranial radiation in small cell lung cancer (Slotman 2007)?
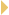 DR JOHNSON: That study observed 286 patients who had extensive-stage
small cell lung cancer.
DR JOHNSON: That study observed 286 patients who had extensive-stage
small cell lung cancer.
Patients were randomly assigned to one of several
different doses of prophylactic cranial radiation. Results showed a threefold
reduction in the primary endpoint of cumulative incidence of symptomatic
brain metastasis.
Even more impressive was that the risk of dying was reduced
by more than 30 percent (1.2).
Select publications

